| [新!]材料与资源 |
| 首页 |
| 研究员 |
| 实验室成员 |
| 研究方向 |
| 部分发表论文 |
| 招聘 |
| 实验室活动 |
| 如何抵达 |
| GRAB探针常见问题 |
部分发表论文
[ 实验室代表性论文 | 实验室代表性综述 | 合作代表性论文 | 主要研究论文 | 综述,书评等 | 预印本 | 合作发表论文]
代表性论文(2018至今)
查看 详细信息

|

|

|

|

|

|

|
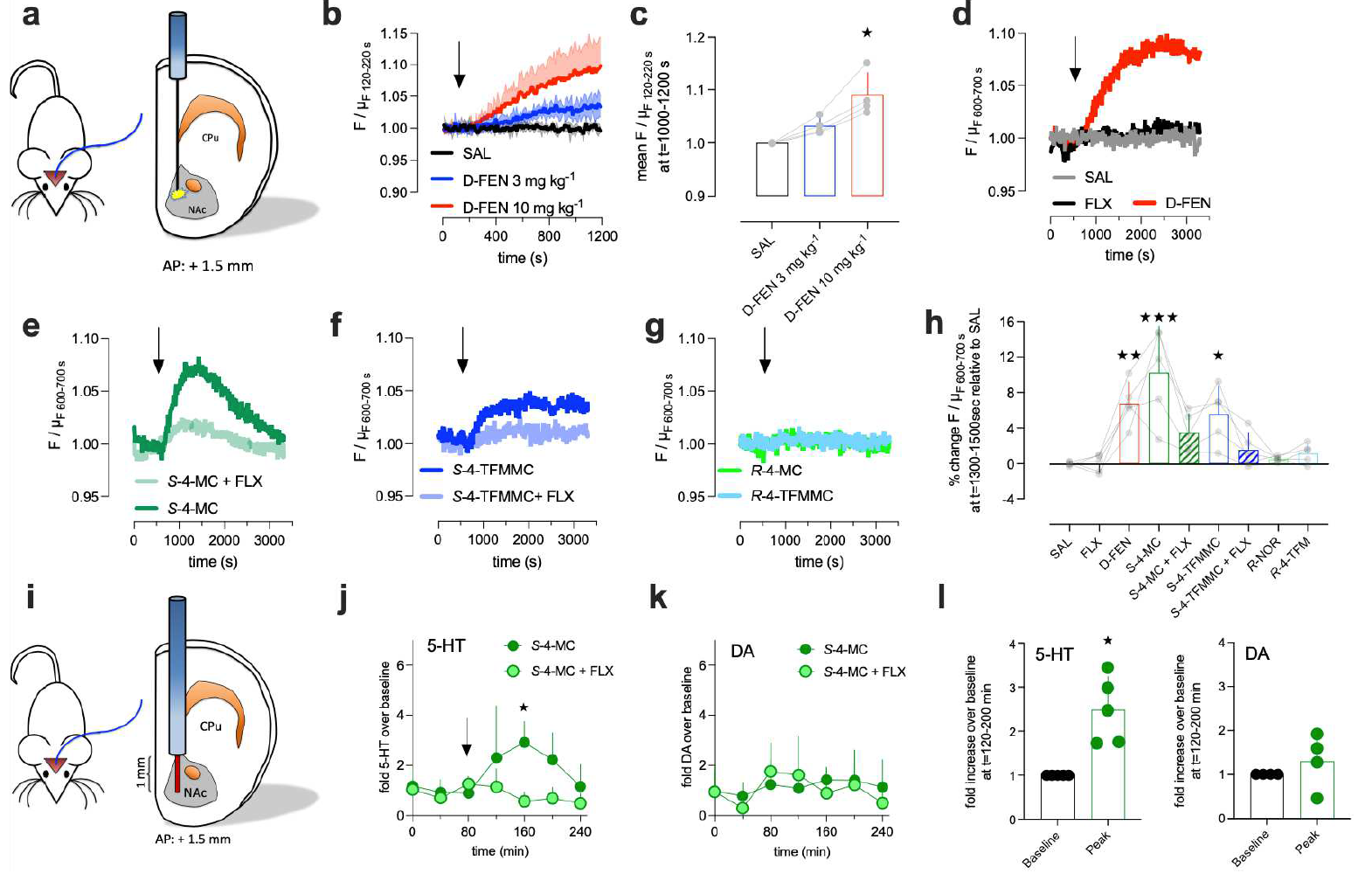
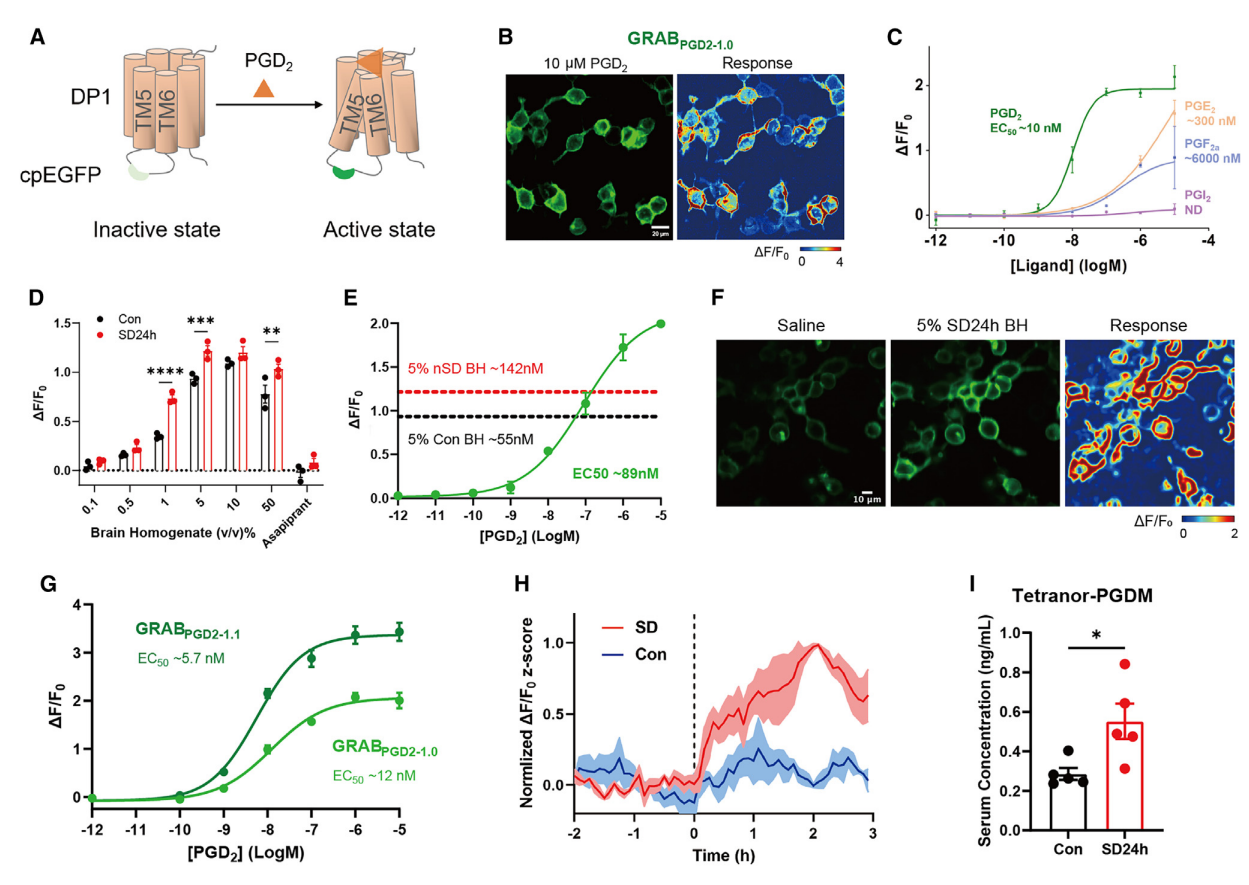
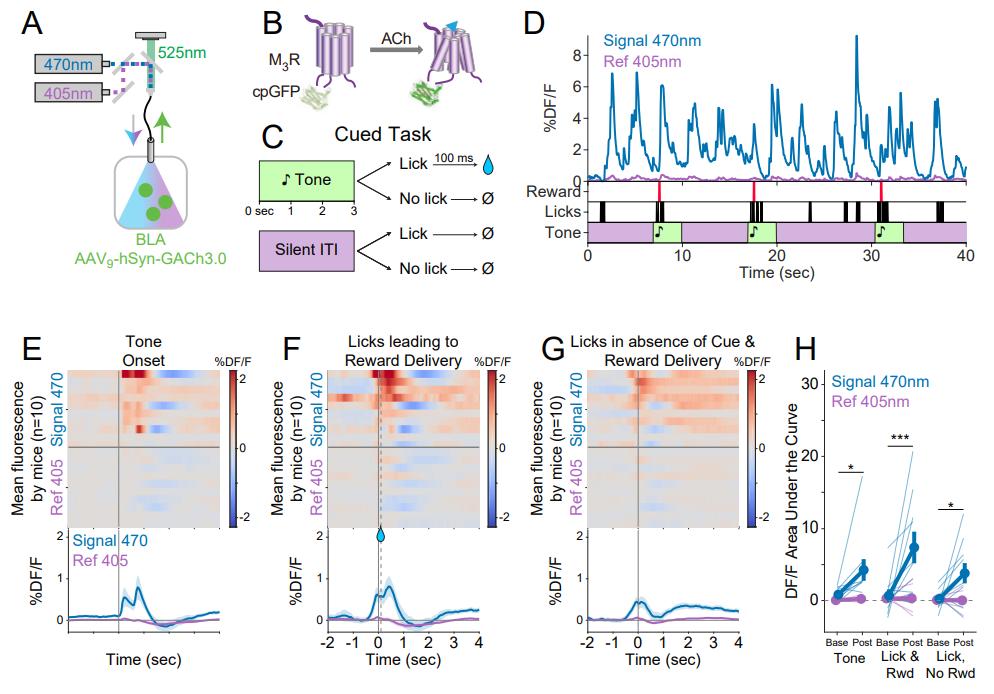
| 远红多巴胺探针应用于多色成像 (2025) | 血清素能嗜铬细胞在肠道保护中的作用 (2025) | 神经肽与神经递质释放的不同调控规律 (2025) | 胆汁淤积瘙痒机制及治疗对策 (2024) | 章鱼胺在厌恶性学习中的作用 (2024) | 新一代去甲肾上腺素探针 (2024) | UDP探针 (2024) |

|

|

|

|

|

|

|
| 新一代五羟色胺探针 (2024) | 新一代多巴胺探针 (2024) | 神经肽探针工具包 (2023) | 腺苷释放机制 (2023) | 第一代组胺探针 (2023) | 五羟色胺在联想学习中的作用 (2023) | 第一代催产素探针 (2023) |

|

|

|

|

|

|

|
|
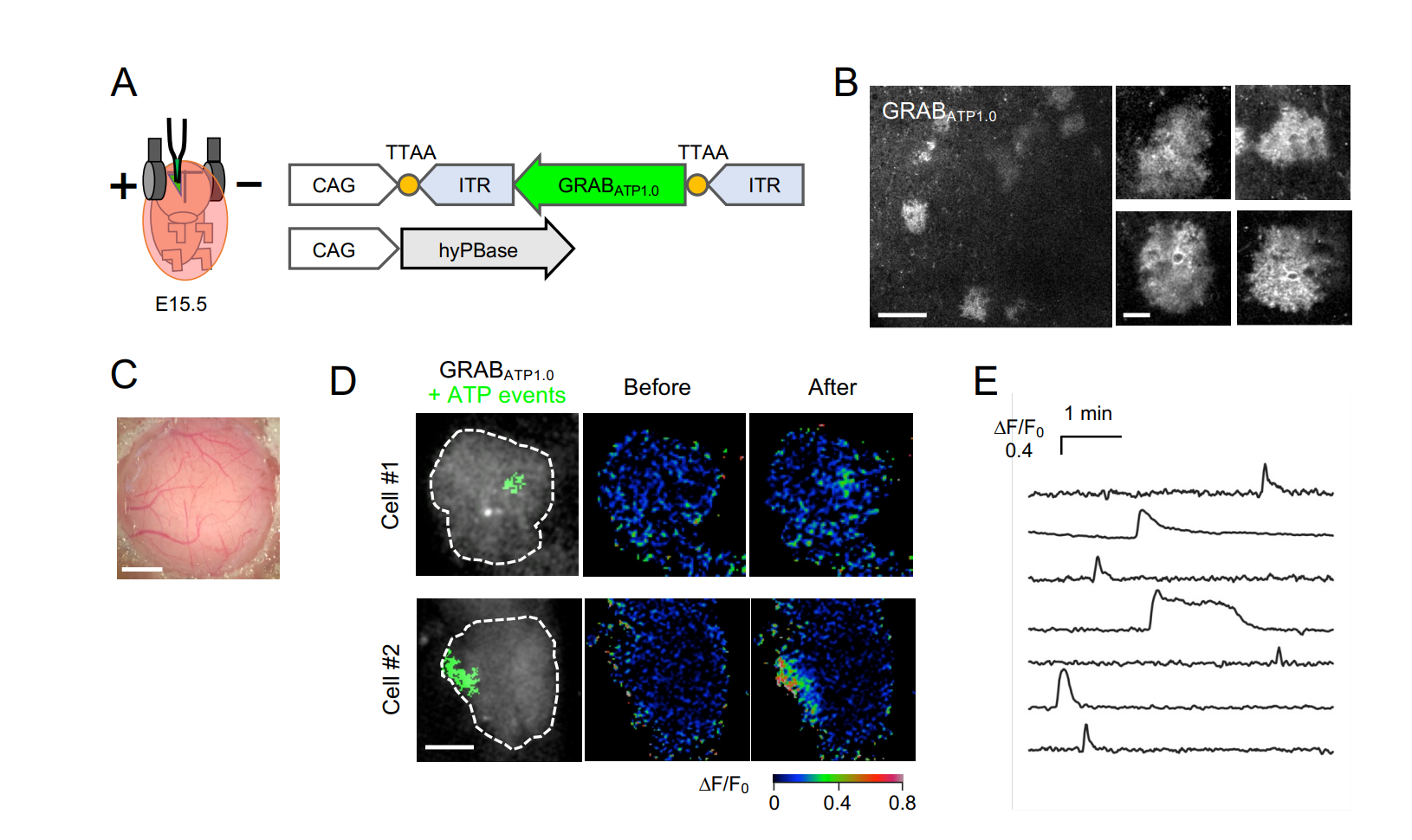
第一代ATP探针 (2022) "Best of Neuron 2021-2022" |
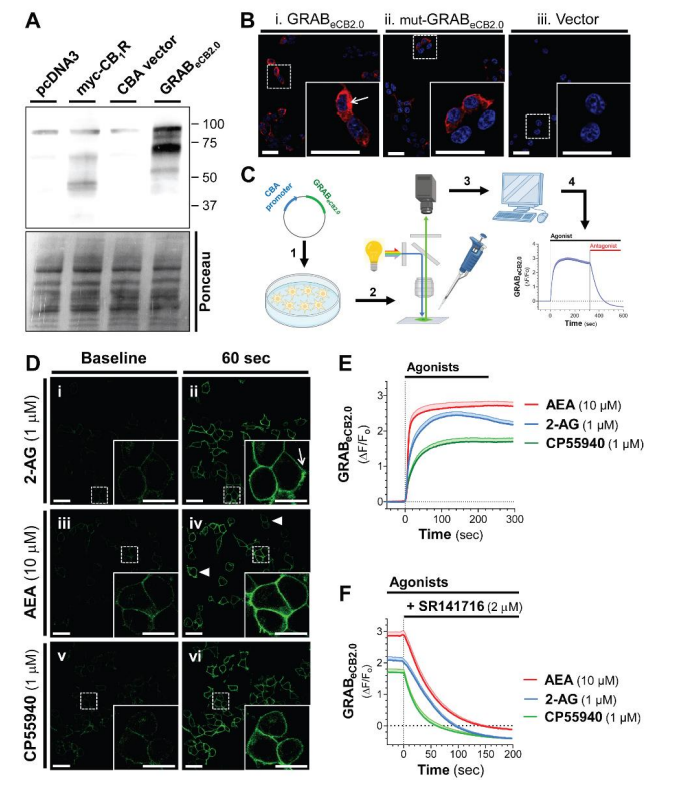
第一代内源大麻素探针 (2021) | UDP-glucose 囊泡转运体 (2021) | 第一代五羟色胺探针 (2021) | 第二代多巴胺探针 (2020) | 第二代乙酰胆碱探针 (2020) | 痒觉机制 (2019) |

|

|

|
|

|
||
| PARIS (2019) | 第一代去甲肾上腺素探针 (2019) |
GRAB sensors设计 策略 (2019) |
第一代多巴胺探针 (2018) | 第一代乙酰胆碱探针 (2018) |
实验室代表性综述(2015至今)
|

|

|

|

|

|

|
| 用红色荧光探针揭示钙钾动力学 (2025) | 基因编码神经调质荧光探针的研究进展与展望 (2025) | 多巴胺探针的原理,应用和未来 (2025) | 宽场成像 (2024) | Saxitoxin的光笼合衍生物 (2024) | 探针在体应用 (2024) | 探针在抑郁症中的应用 (2024) |

|

|

|

|
|||
| 检测DA的工具 (2023) | 检测神经肽的方法 (2023) | GRAB探针综述 (2022) | 神经生物学的吹笛人 (2015) |
合作代表性论文(2018至今)
主要研究论文
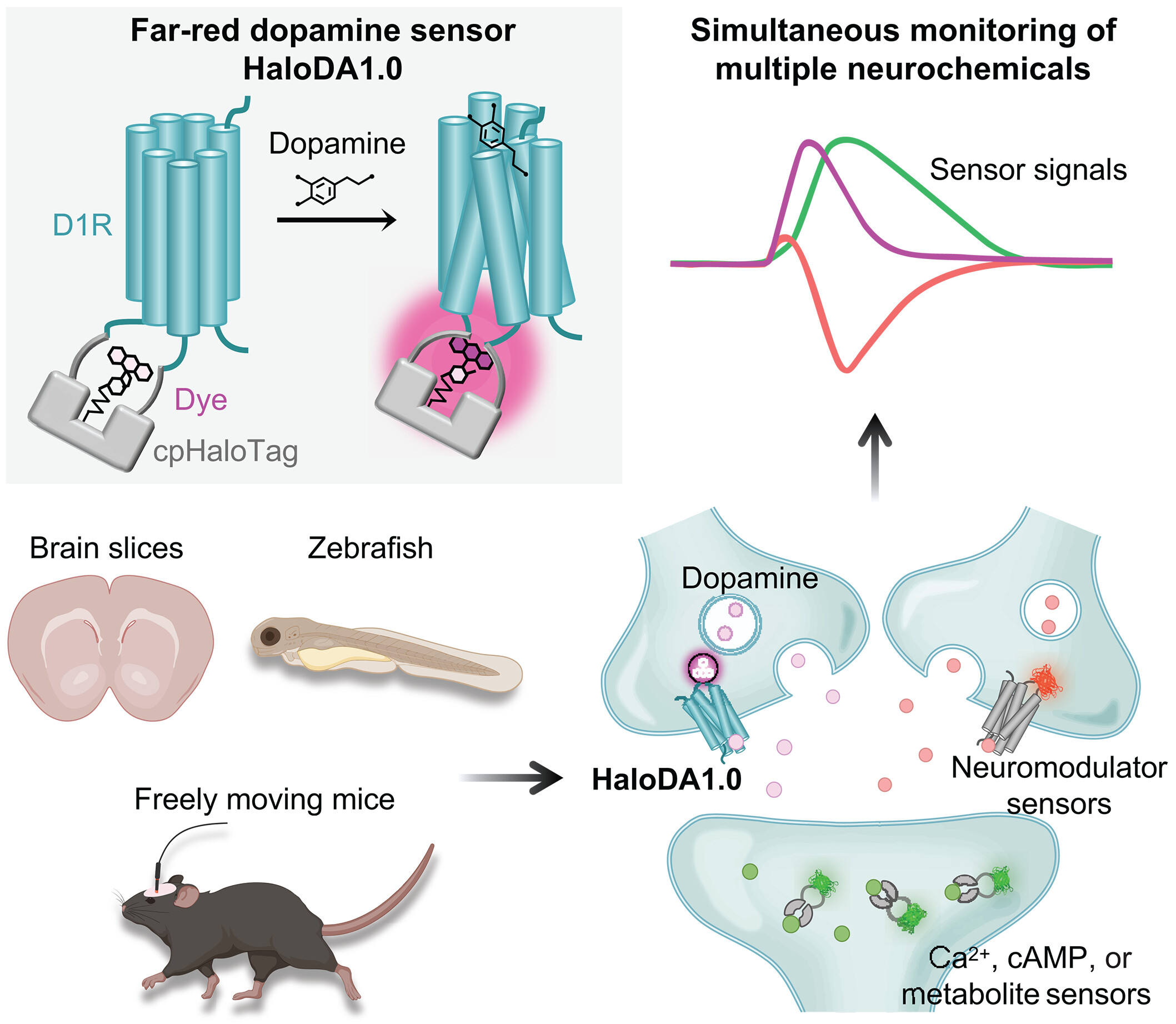 |
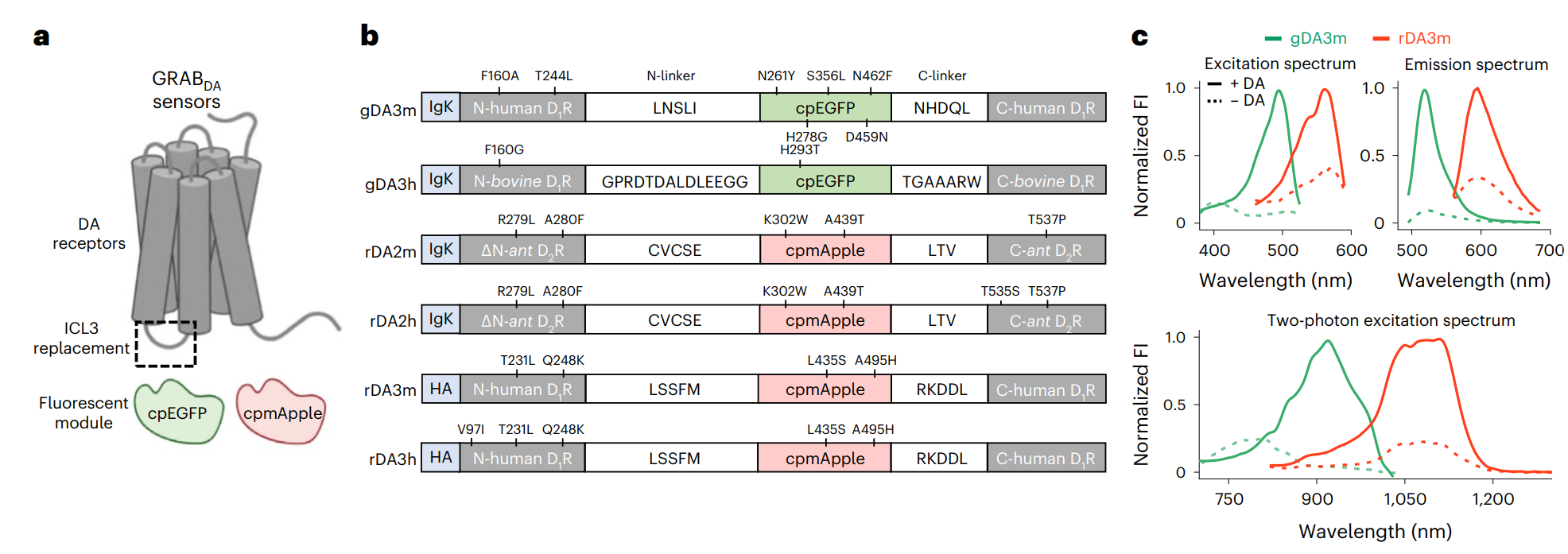
· Zheng, Y.#, Cai, R.#, Wang, K., Zhang, W., Zhuo, Y., Dong, H., Zhang, Y., Wang, Y., Deng, F., Ji, E., Cui, Y.
Fang, S., Zhang, X., Huang, H., Zhang K., Wang J., Li, G., Miao, X., Wang, Z., Yang, Y., Li, S., Grimm, J., Johnsson, K., Schreiter, E., Lavis, L., Chen, Z., Mu, Y., & Li, Y.* (2025)
In vivo multiplex imaging of dynamic neurochemical networks with designed far-red dopamine sensors.
Science.
[Full Text]
[PDF] See also Research highlights by Zheng, Y.* (2025) A far-red dopamine sensor unlocks multiplex views of in vivo neuromodulation. [Full Text] [PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2024.12.22.629999v1 |
|
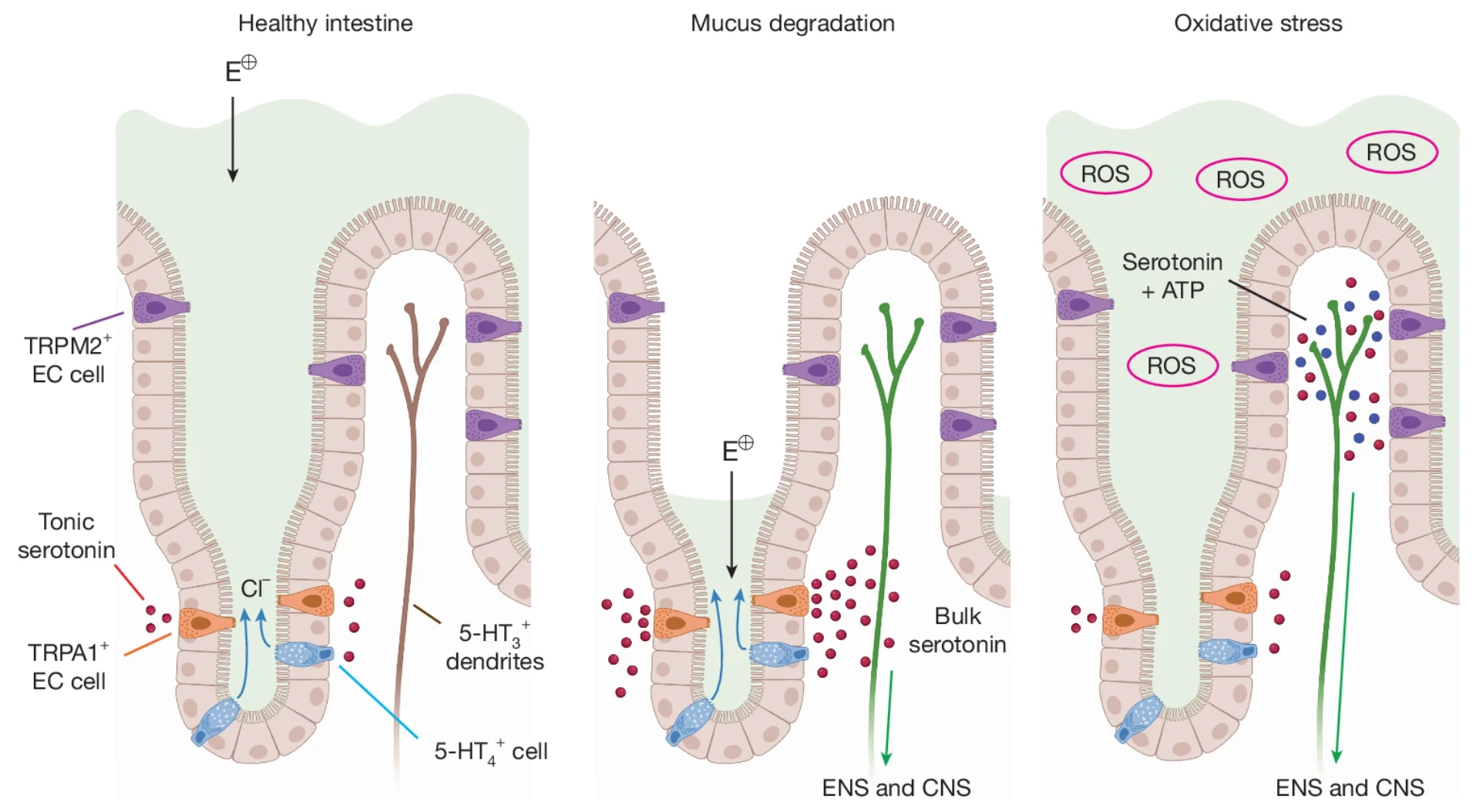 |
· K. Touhara*, K., D. Rossen, D., Deng, F., Castro, J., M. Harrington. A., Chu, T., Garcia-Caraballo, S., Brizuela, M., O'Donnell, T., Xu, J., Cil, O., M. Brierley, S.*, Li, Y.* & Julius, D.* (2025)
Topological segregation of stress sensors along the gut crypt-villus axis.
Nature.
[Full Text]
[PDF] |
|
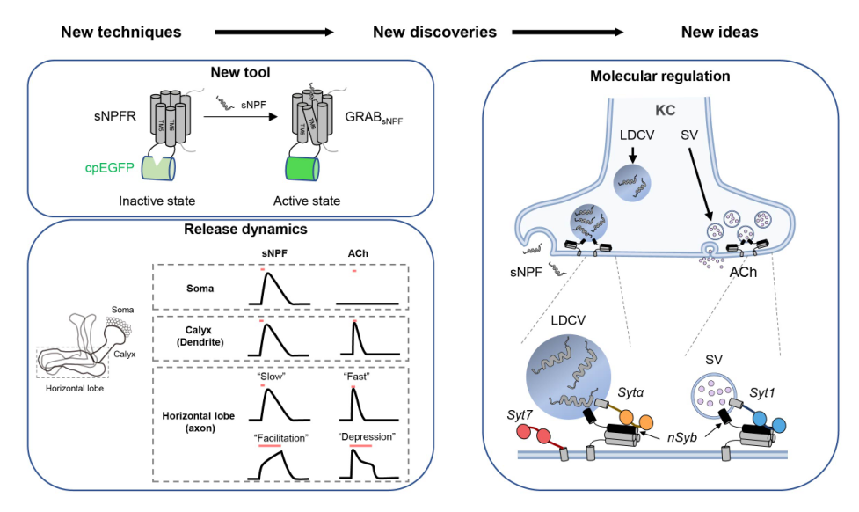 |
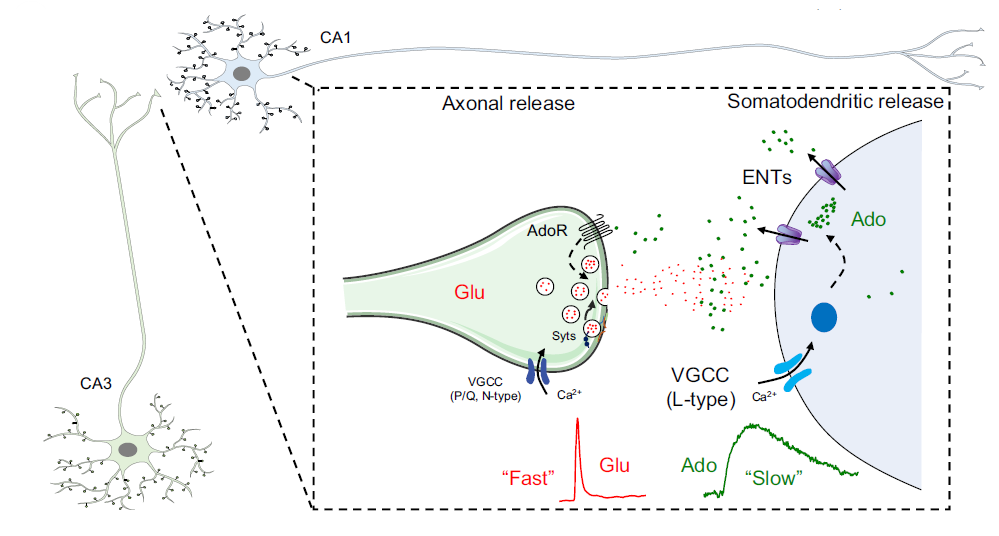
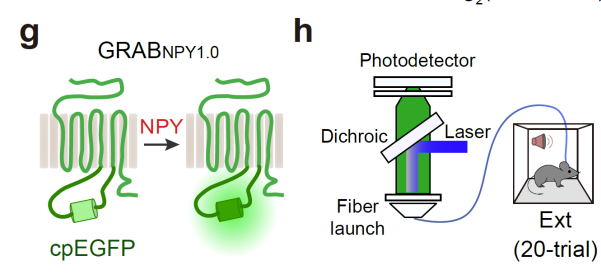
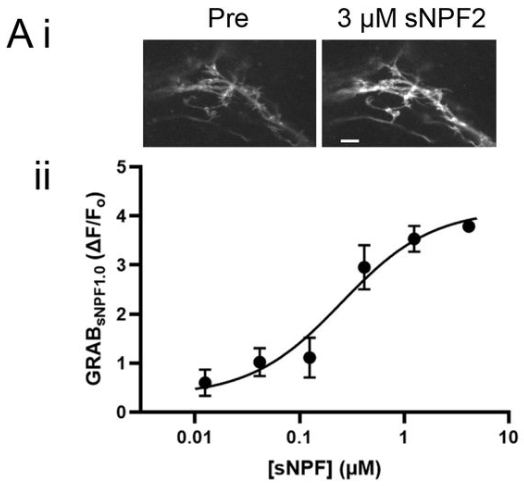
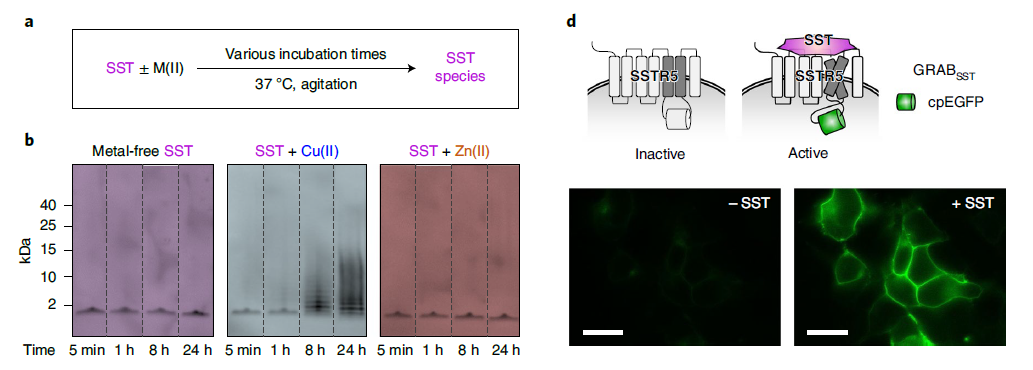
· Xia, X., & Li, Y.* (2025) A high-performance GRAB sensor reveals differences in the dynamics and molecular regulation between neuropeptide and neurotransmitter release.
Nature Communications.
[Full Text]
[PDF]
[Supplemental Data] See also BioRxiv https://www.biorxiv.org/content/10.1101/2024.05.22.595424v1 |
|
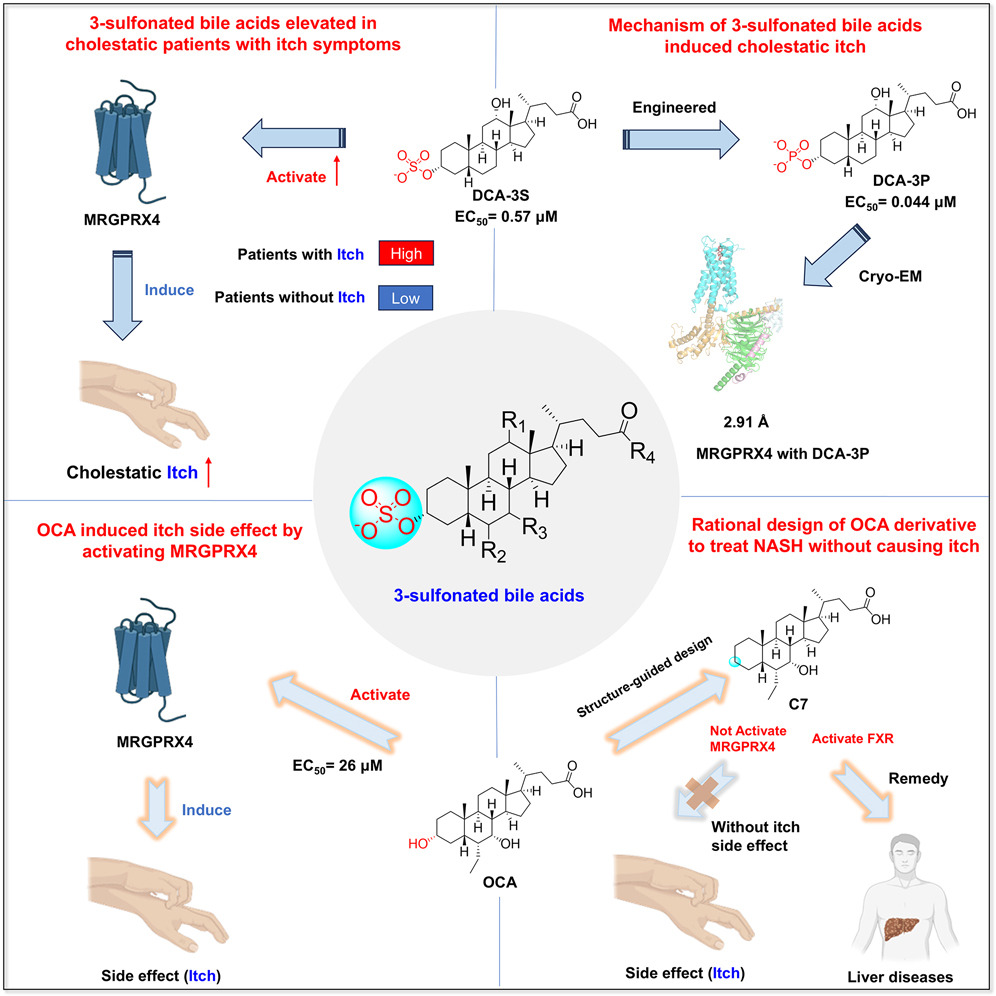 |
· Yang J.#, Zhao, T.#, Fan, J.#, Zou, H.#, Lan, G., Guo, F., Shi, Y., Ke, H., Yu, H., Yue, Z., Wang, X., Bai Y., Li, S., Liu, Y., Wang, X., Chen, Y.*, Li, Y.*, & Lei, X.* (2024) Structure-guided discovery of bile acid derivatives for treating liver diseases without causing itch.
Cell. Volume 187, Issue 25.
[Full Text]
[PDF]
[Supplemental Data] |
|
 |
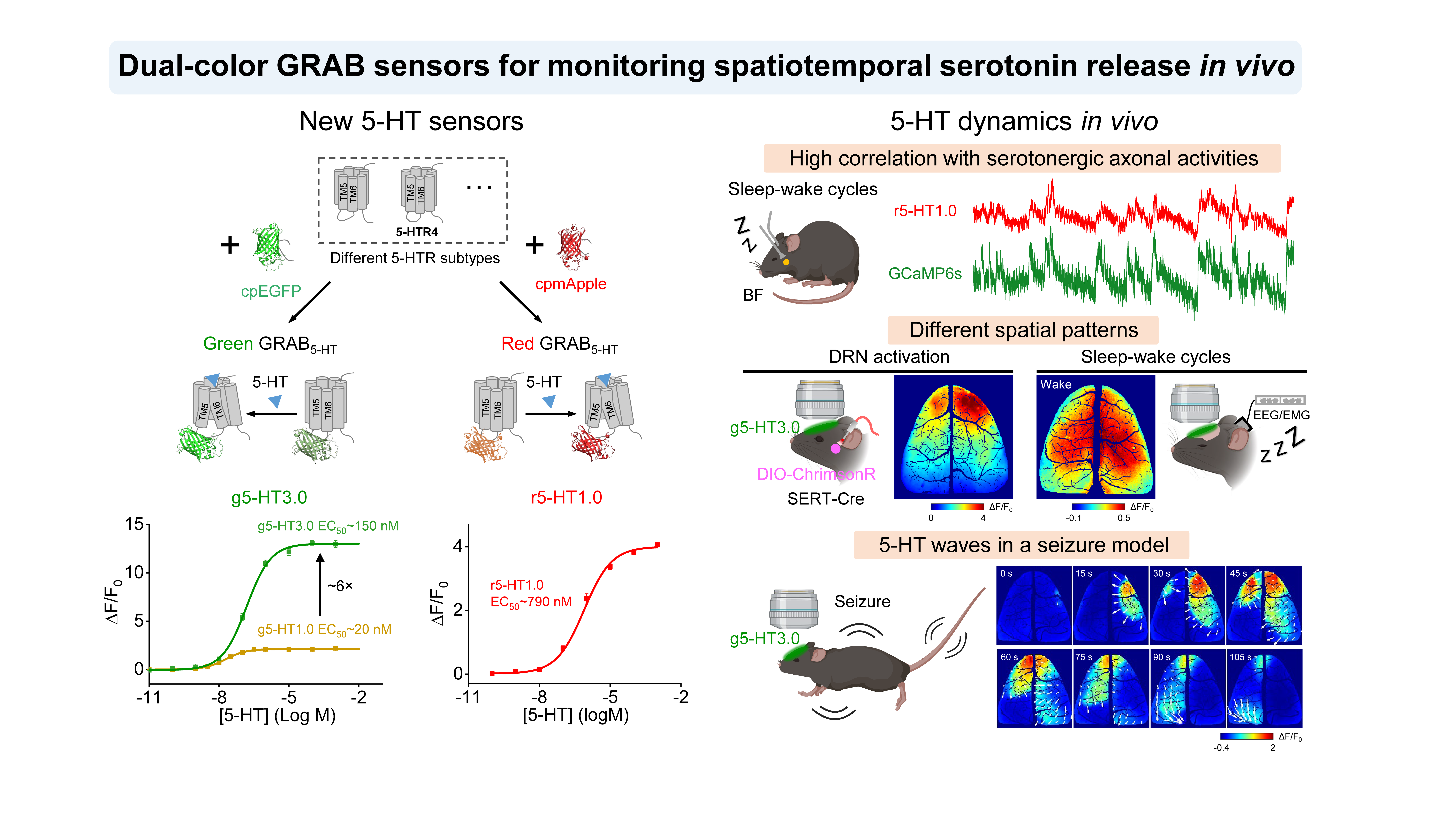
· Lv, M., Cai, R., Zhang, R., Xia, X.,
Li, X., Wang, Y., Wang, H., Zeng, J., Xue, Y., Mao, L., & Li, Y.* (2024).
An octopamine-specific GRAB sensor reveals a monoamine relay circuitry that boosts aversive learning.
National Science Review. 11(5): nwae112.
[Full Text]
[PDF]
[Supplemental Data] See also BioRxiv https://doi.org/10.1101/2024.03.09.584200 |
|
 |
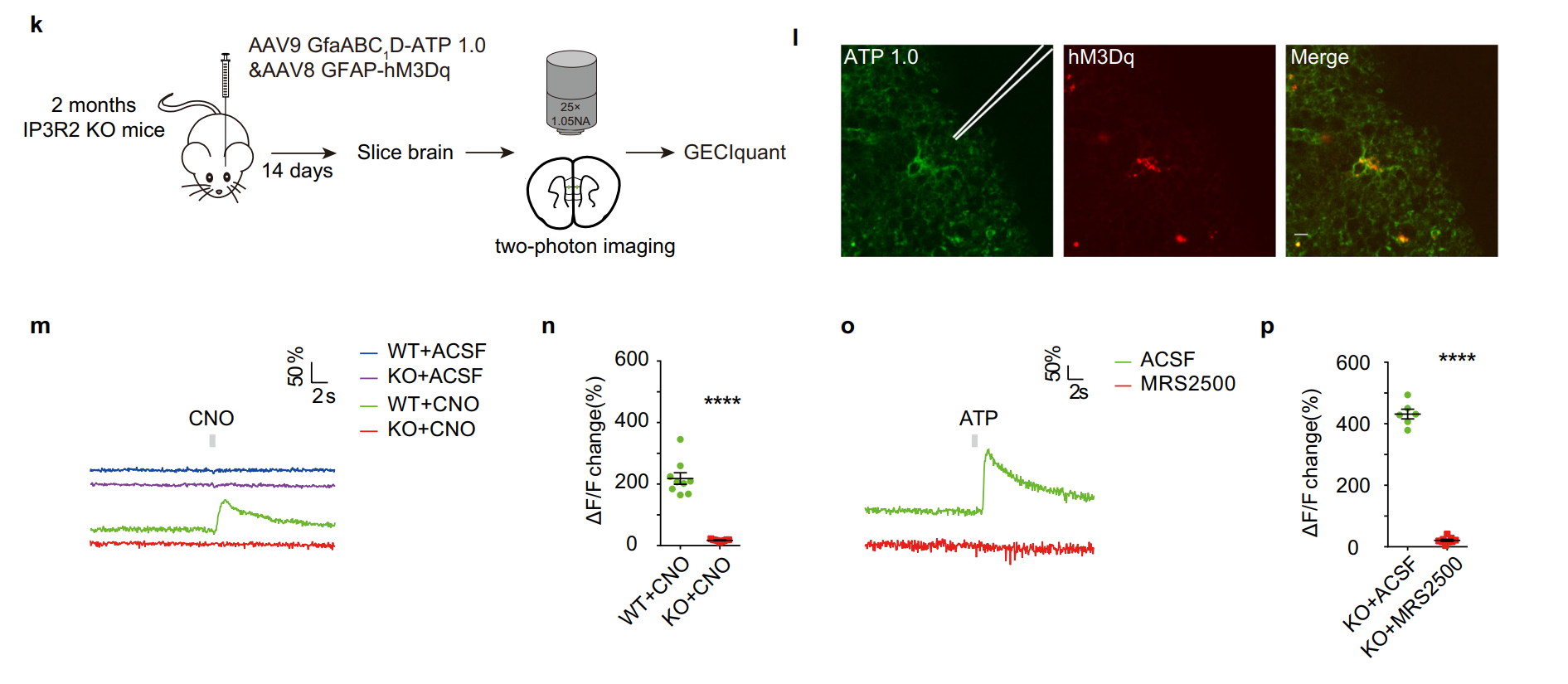
· Umpierre, A. D.#*, Li, B.#, Ayasoufi, K., Simon, W. L.,
Zhao, S., Xie, M., Thyen, G., Hur, B., Zheng, J., Liang, Y., Bosco, D. B., Maynes, M. A., Wu, Z.,
Yu, X., Sung, J., Johnson, A. J., Li, Y.*, & Wu, L.-J.* (2024)
Microglial P2Y6 calcium signaling promotes phagocytosis and shapes neuroimmune
responses in epileptogenesis.
Neuron. 112(12): 1959-1977. e10.
[Full Text]
[PDF]
[Supplemental Data]
[UDP Release Dynamics ex vivo]
[UDP Release Dynamics in vivo] See also BioRxiv https://www.biorxiv.org/content/10.1101/2023.06.12.544691v1 |
|
 |
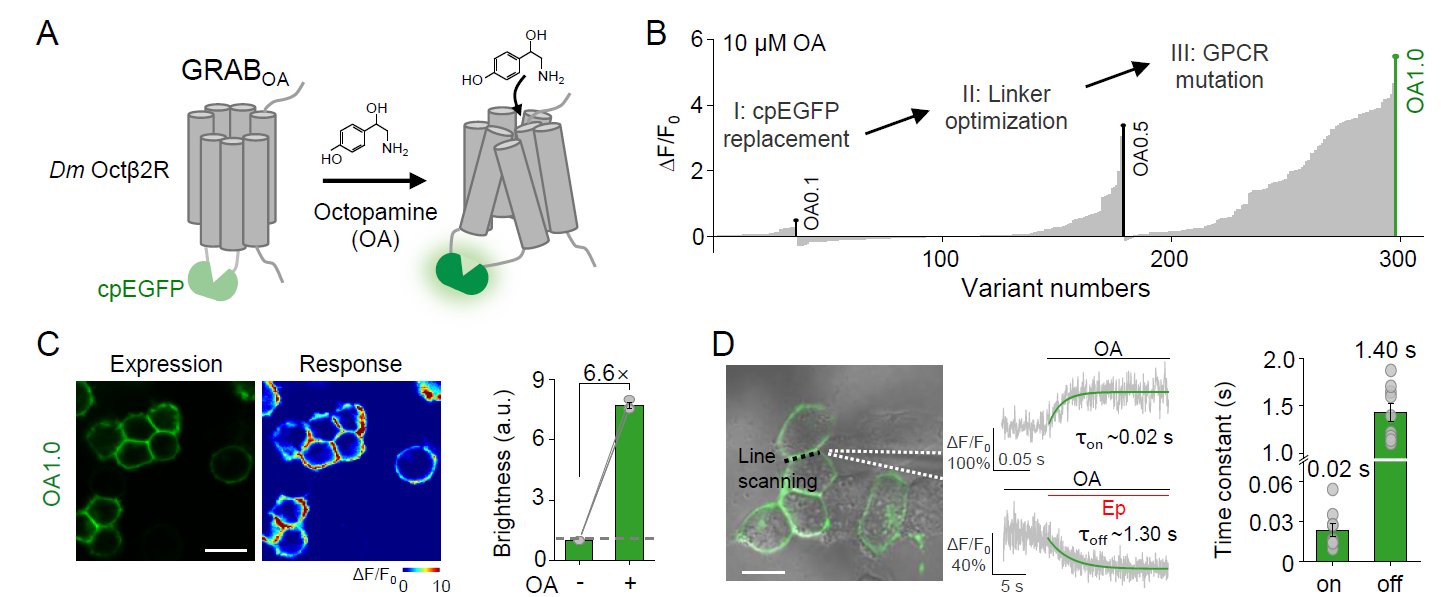
· Feng, J.*, Dong, H., Lischinsky, J. E., Zhou, J., Deng, F.,
Zhuang, C., Miao, X., Wang, H., Li, G., Cai, R., Xie, H., Cui, G., Lin, D.,
& Li, Y.* (2024).
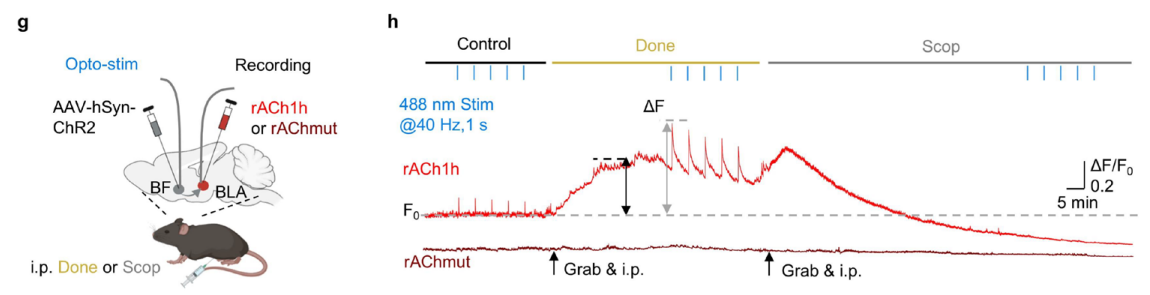
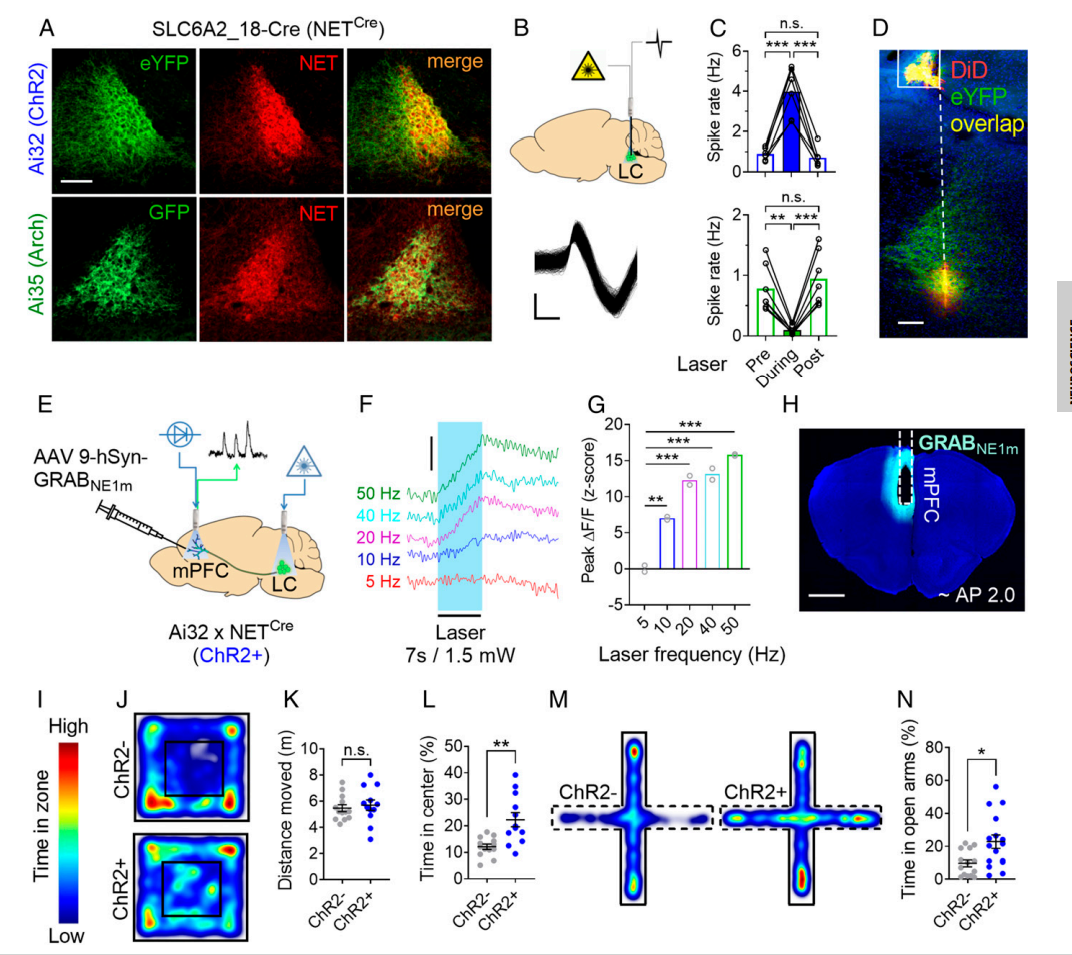
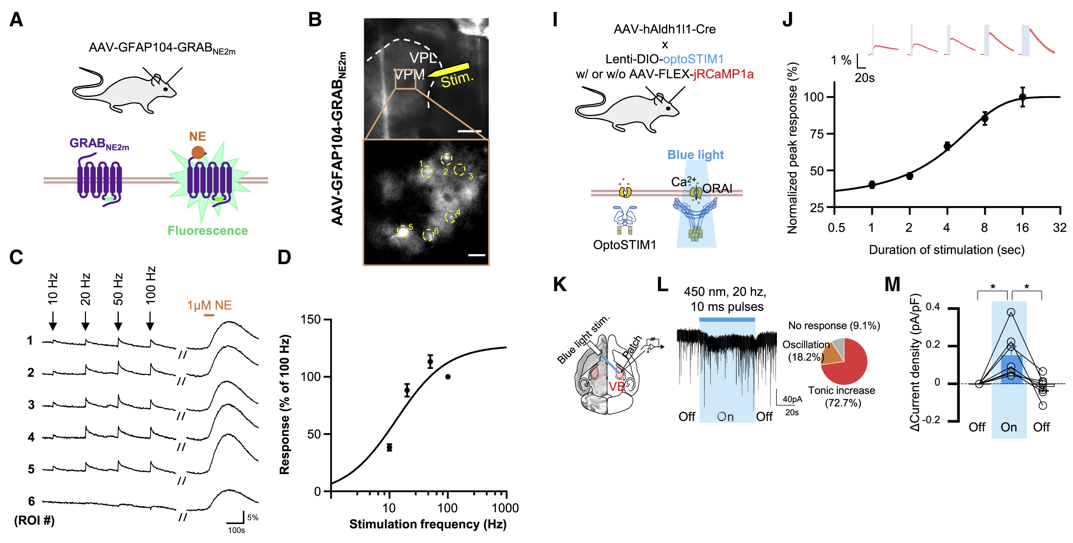
Monitoring norepinephrine release in vivo using next-generation GRABNE sensors.
Neuron. 112(12): 1930-1942. e6.
[Full Text]
[PDF]
[Supplemental Data] See also BioRxiv https://doi.org/10.1101/2023.06.22.546075 |
|
 |
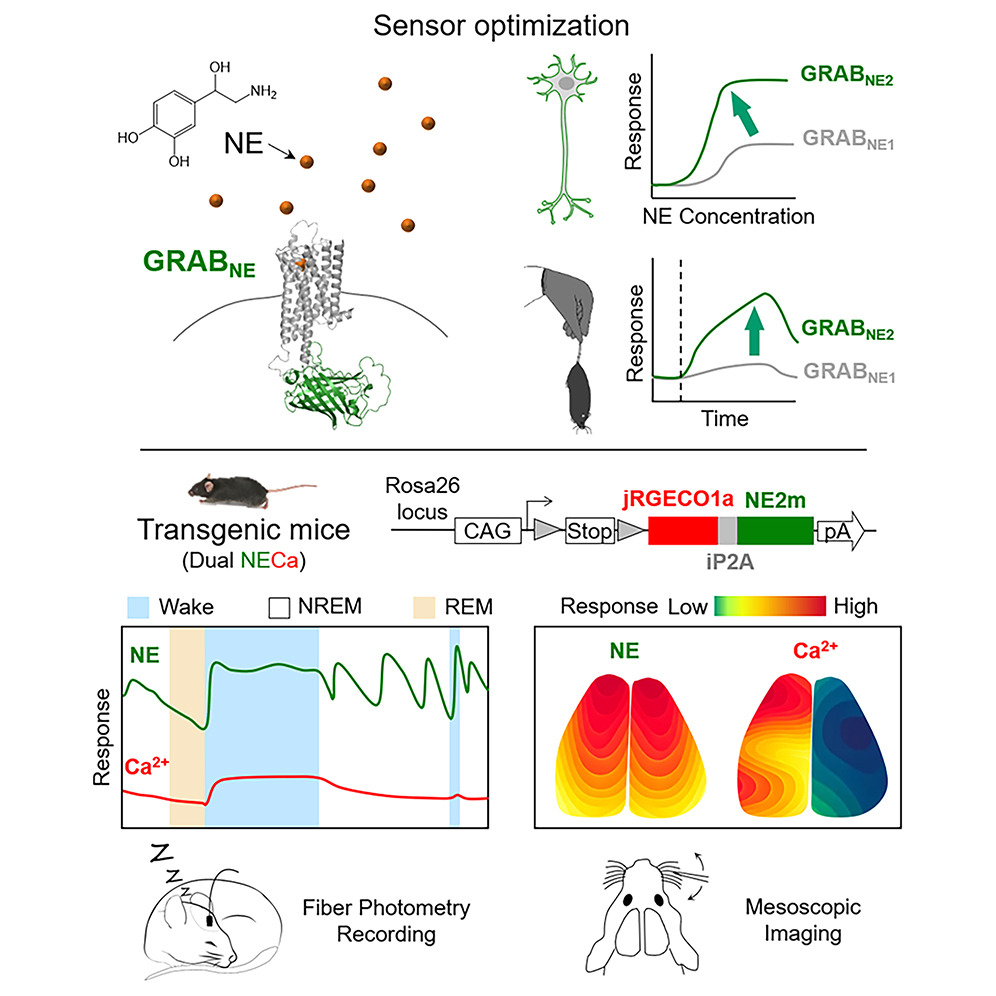
· Deng, F.#, Wan, J.#, Li, G., Dong, H., Xia, X., Wang, Y., Li, X., Zhuang, C., Zheng, Y., Liu, L., Yan, Y., Feng, J., Zhao, Y., Xie, H., & Li, Y.*(2024).
Improved green and red GRAB sensors for monitoring spatiotemporal serotonin release in vivo.
Nature Methods. 21(4): 692-702.
[Full Text]
[PDF]
[Supplemental Data]
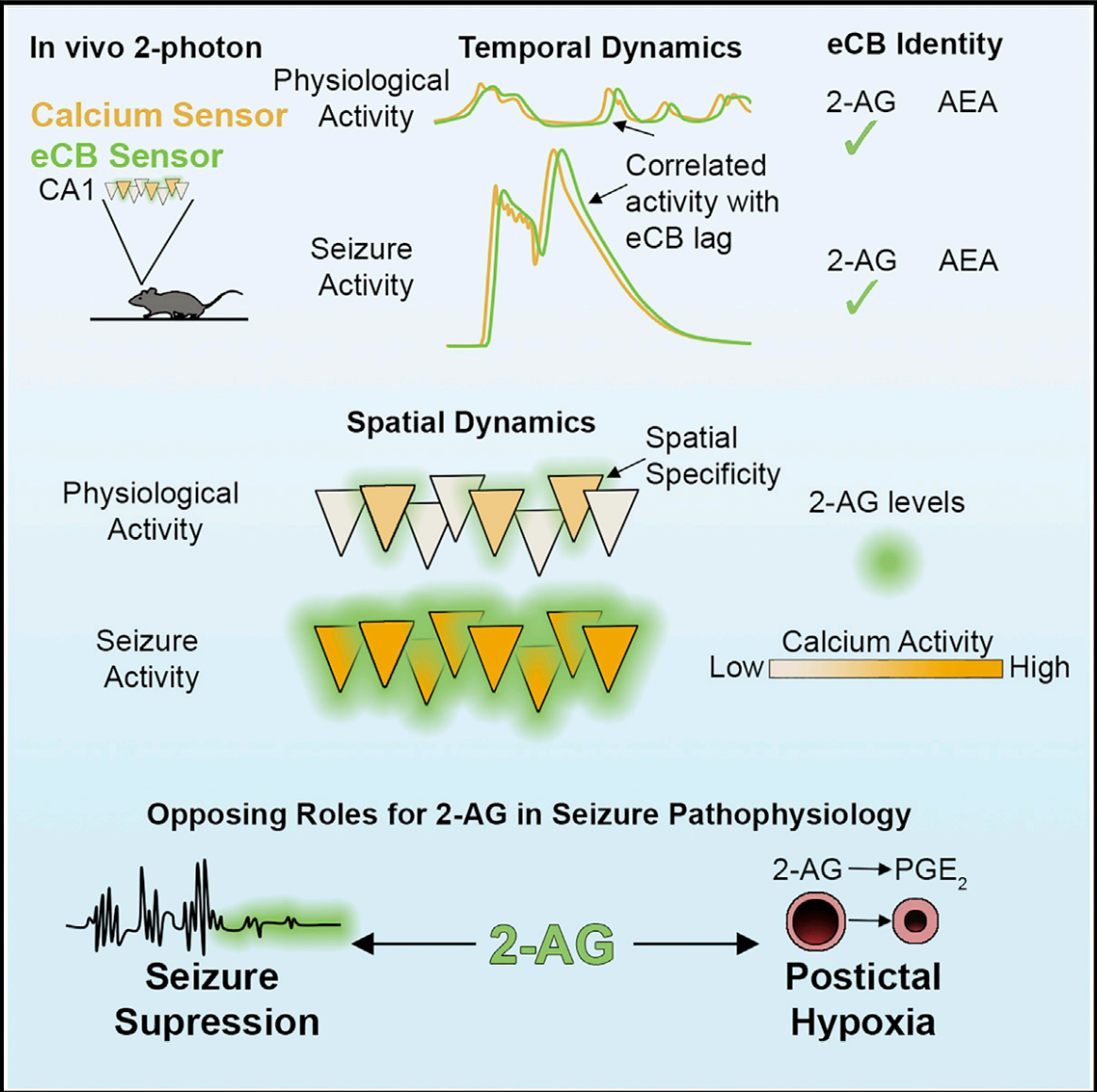
[5-HT and Ca2+ Waves During Seizure]
[5-HT and eCB Waves During Seizure] See also BioRxiv https://www.biorxiv.org/content/biorxiv/early/2023/05/30/2023.05.27.542566.full.pdf |
|
 |
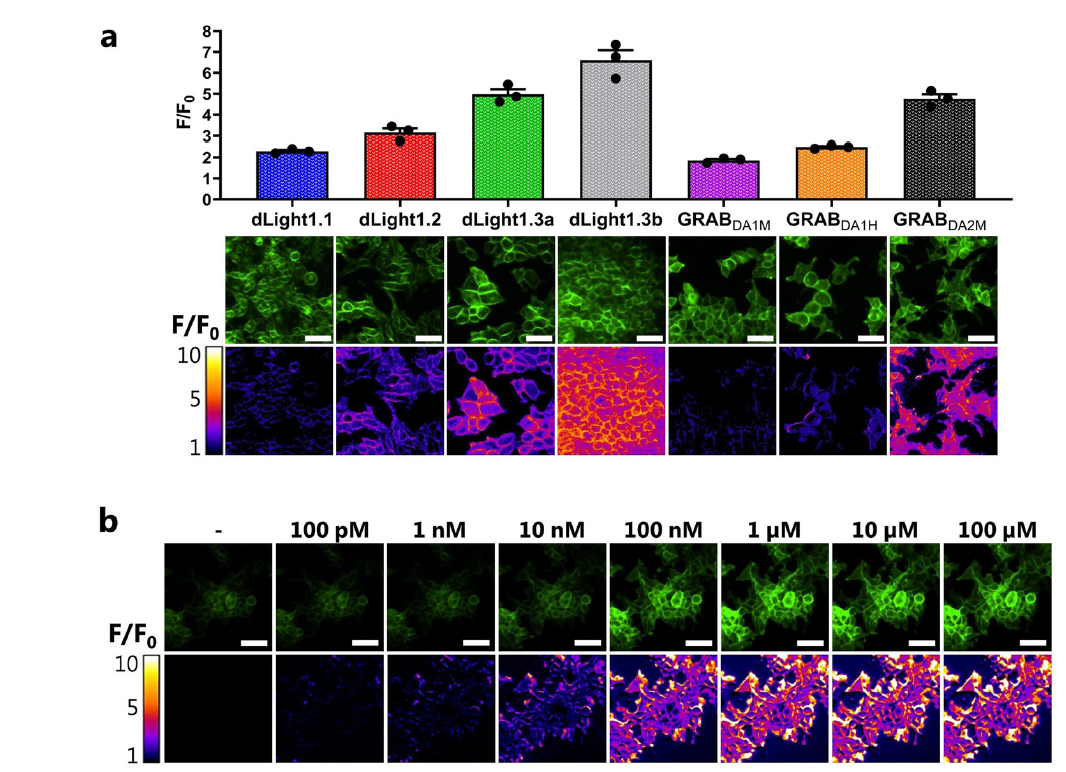
· Zhuo, Y.#, Luo, B.#, Yi, X., Dong, H.,
Miao, X., Wan, J., Williams, J. T., Campbell, M. G., Cai, R., Qian, T.,
Li, F., Weber, S. J., Wang, L., Li, B., Wei, Y., Li, G., Wang, H.,
Zheng, Y., Zhao, Y., Wolf, M. E., Zhu, Y., Watabe-Uchida, M., &
Li, Y.* (2024).
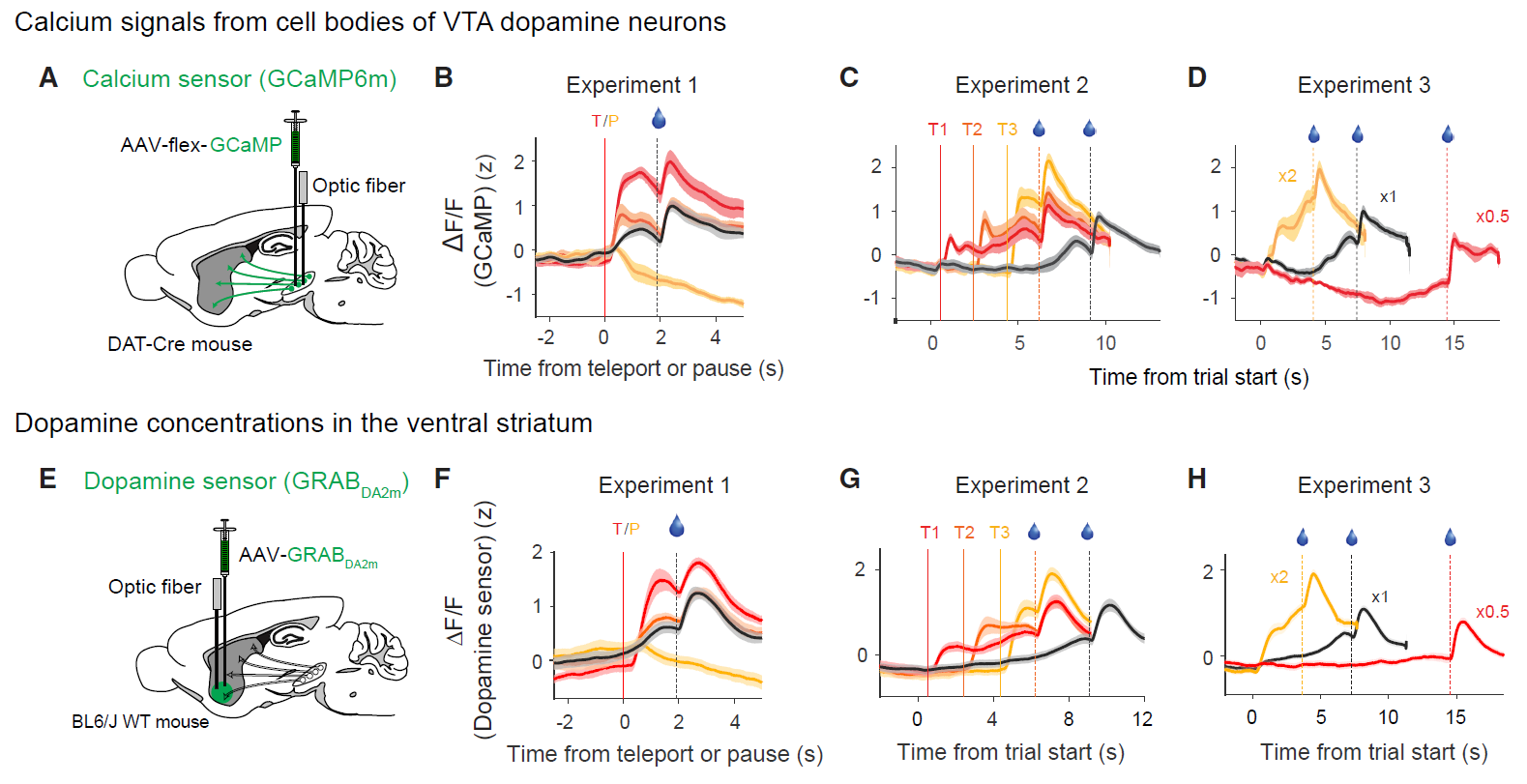
Improved green and red GRAB sensors for monitoring dopaminergic activity in vivo.
Nature Methods. 21(4): 680-691.
[Full Text]
[PDF]
[Supplemental Data] See also BioRxiv https://www.biorxiv.org/content/10.1101/2023.08.24.554559v1 |
|
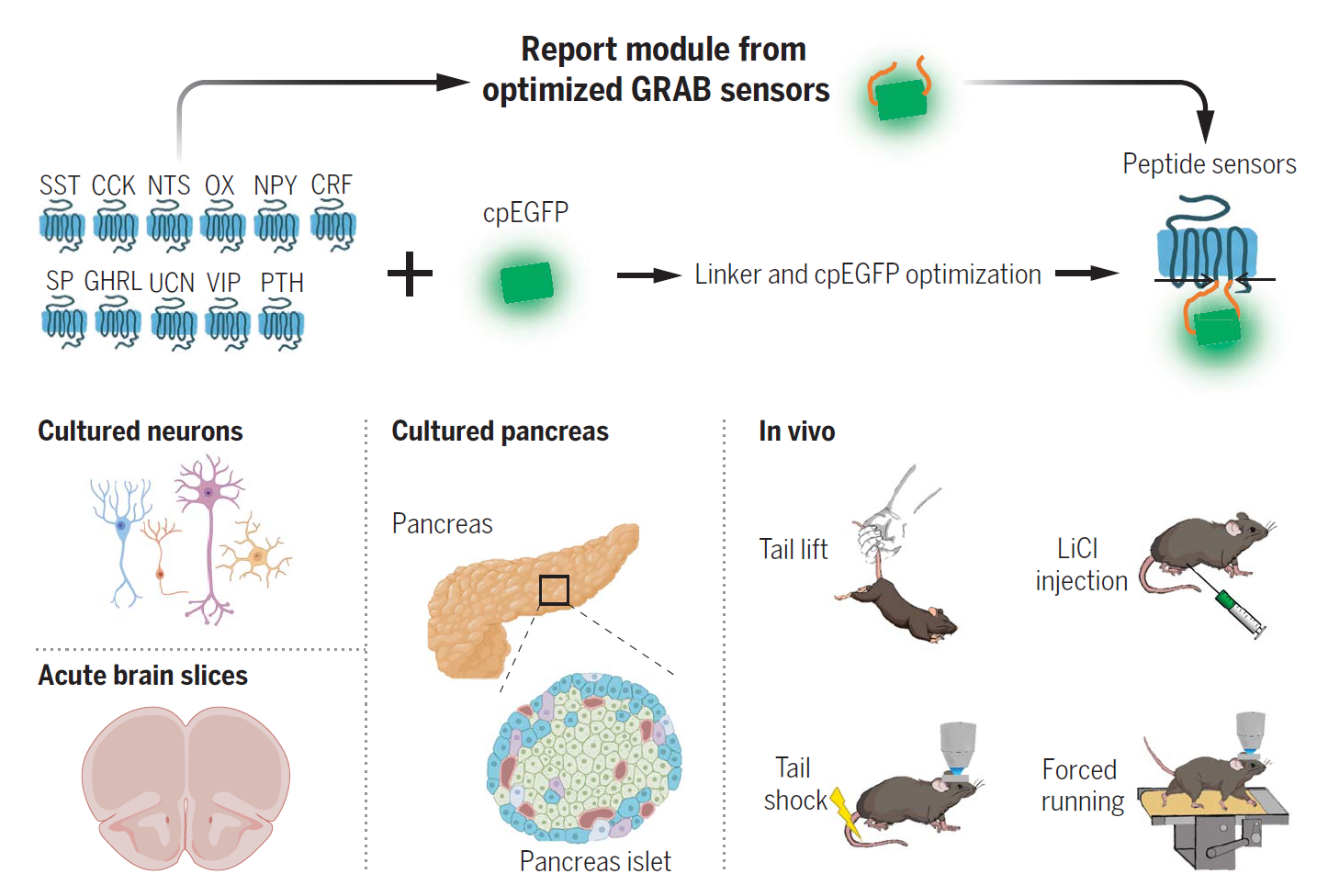 |
·
Wang, H.#, Qian, T.#, Zhao, Y., Zhuo, Y., Wu, C., Osakada, T.,
Chen, P., Chen, Z., Ren, H., Yan, Y., Geng, L., Fu, S.,
Mei, L., Li, G., Wu, L., Jiang, Y., Qian, W., Zhang, L., Peng, W., Xu, M., Hu, J.,
Jiang, M., Chen, L., Tang, C., Zhu, Y., Lin, D., Zhou, J.-N., & Li, Y.* (2023).
A tool kit of highly selective and sensitive genetically encoded neuropeptide sensors.
Science , 382(6672), eabq8173.
[Full Text]
[PDF]
[Supplemental Data]
[Distinct SST Spatial Signal Patterns] See also BioRxiv https://doi.org/10.1101/2022.03.26.485911 |
|
 |
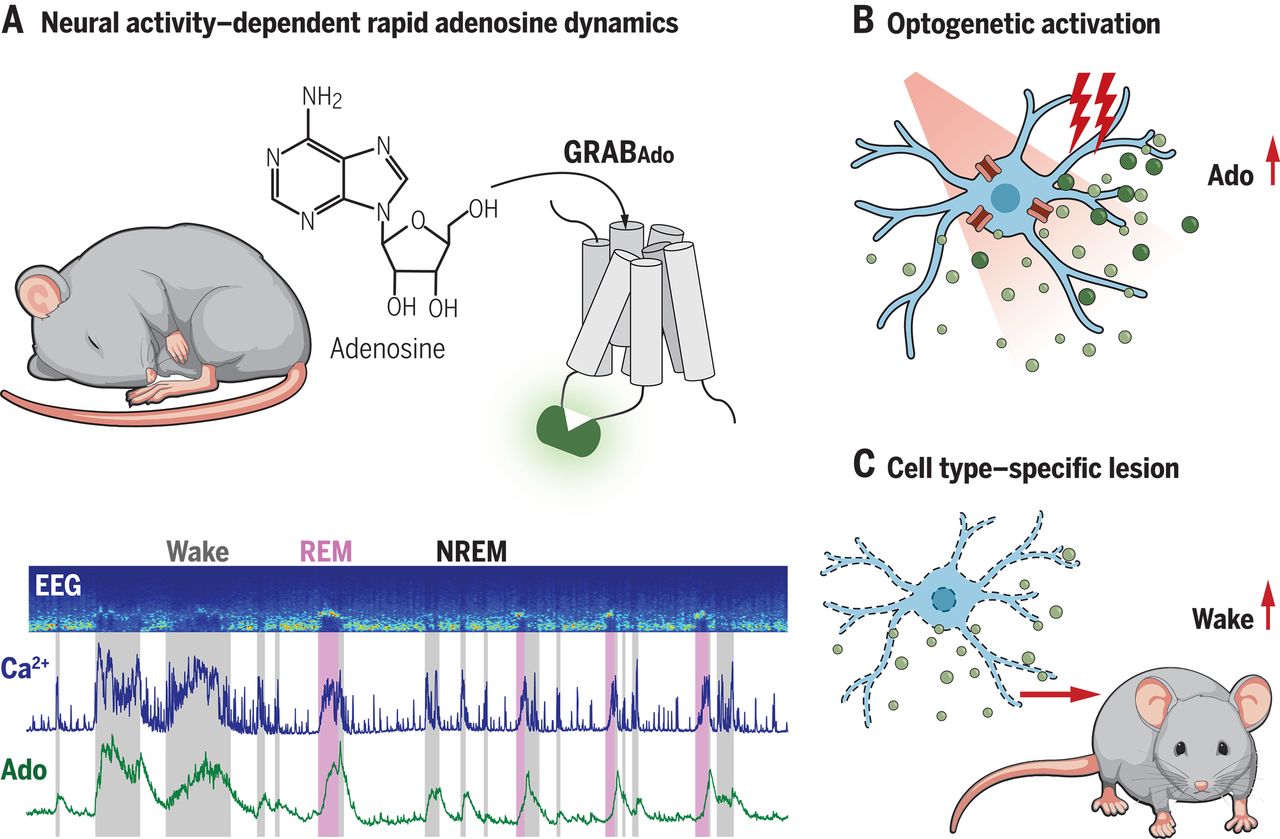
· Wu, Z.#, Cui, Y.#, Wang, H.#, Wu, H., Wan, Y.,
Li, B., Wang, L., Pan, S., Peng, W., Dong, A., Yuan, Z., Jing, M.,
Xu, M., Luo, M.*, & Li, Y.* (2023).
Neuronal activity-induced, equilibrative nucleoside transporter-dependent,
somatodendritic adenosine release revealed by a GRAB sensor.
Proceedings of the National Academy of Sciences, 120(14), e2212387120.
[Full Text]
[PDF]
[Supplemental Data] See also BioRxiv https://www.biorxiv.org/content/10.1101/2020.05.04.075564 |
|
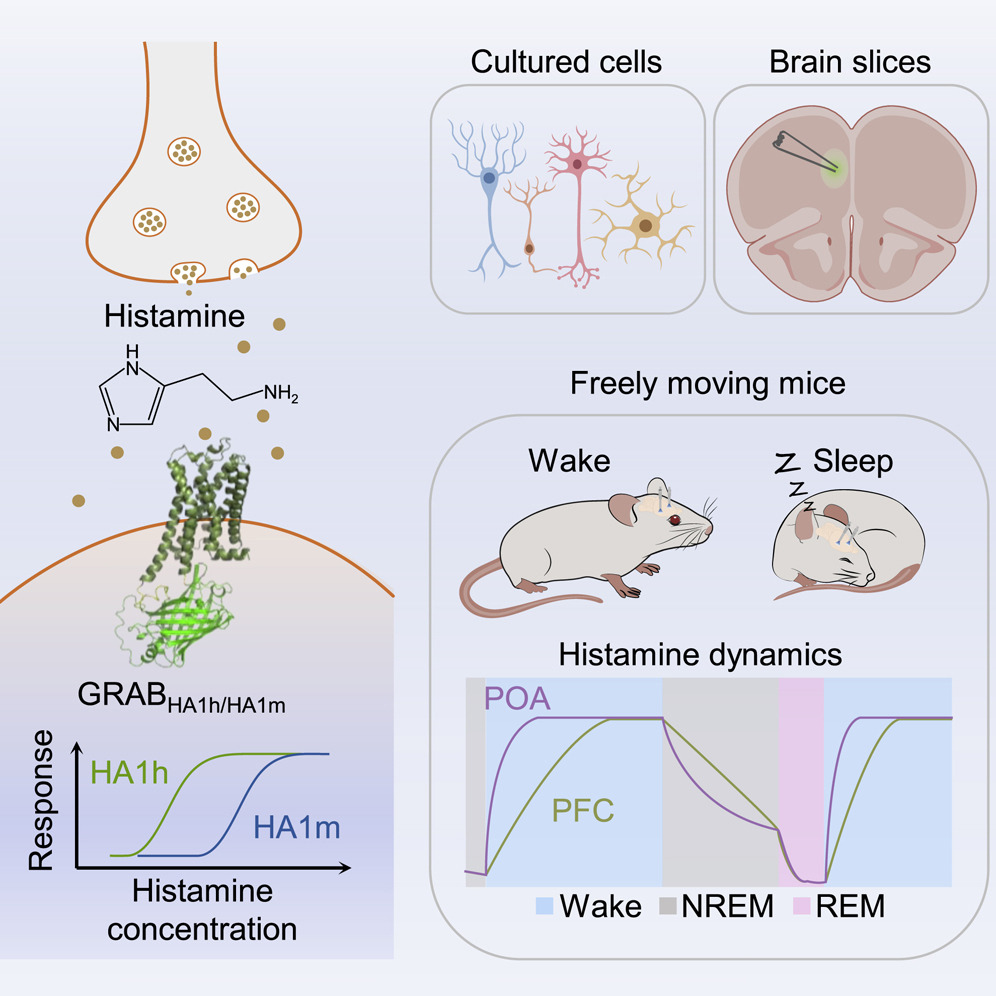 |
· Dong, H.#, Li, M.#, Yan, Y., Qian, T., Lin, Y., Ma, X., Vischer, H. F., Liu, C., Li, G., Wang, H., Leurs, R., & Li, Y.* (2023).
Genetically encoded sensors for measuring histamine release both in vitro and in vivo.
Neuron. 111(10): 1564-1576. e6.
[Full Text]
[PDF]
[Supplemental Data] See also BioRxiv https://doi.org/10.1101/2022.08.19.504485 |
|
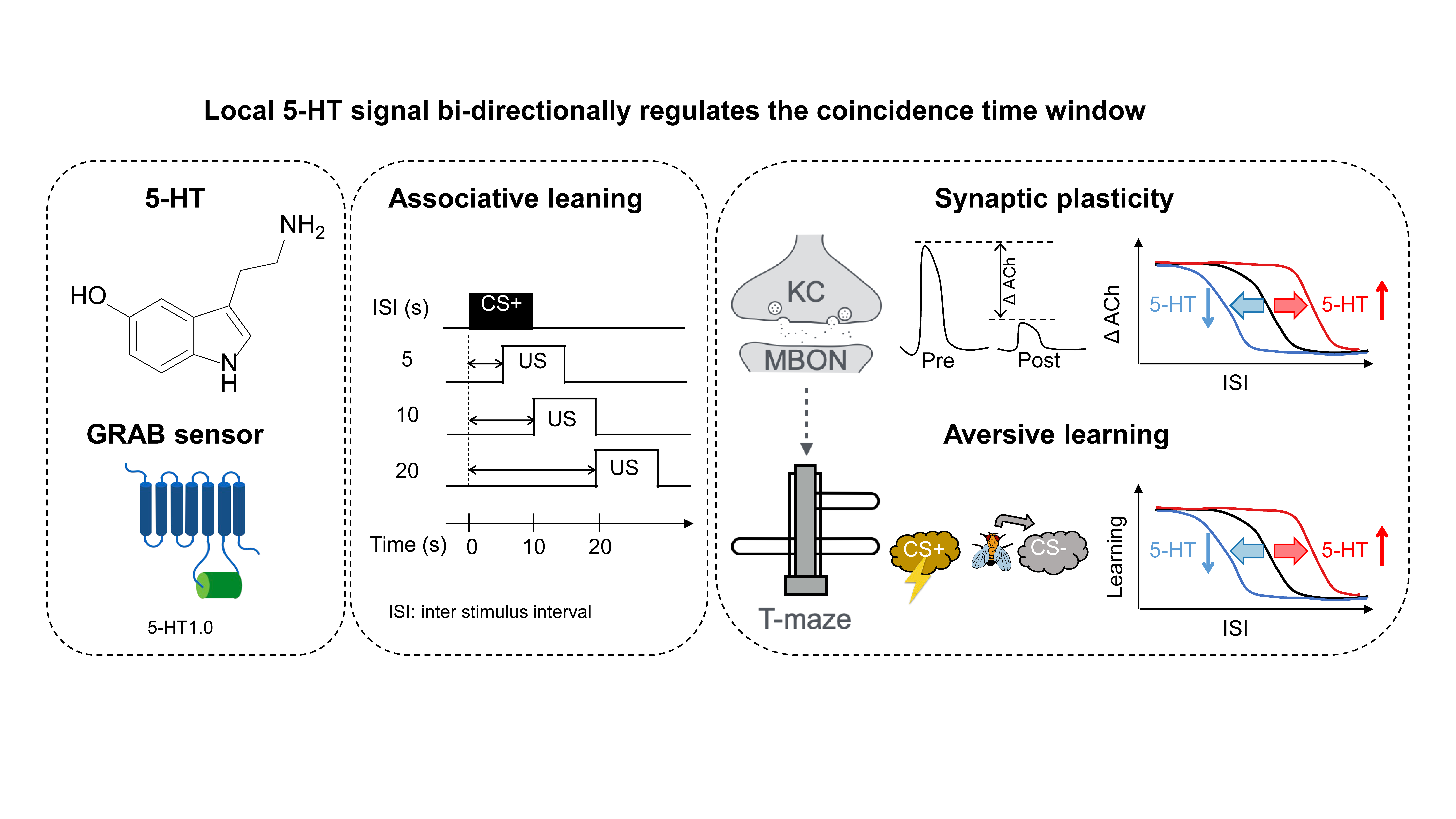 |
· Zeng, J.#*, Li, X.#, Zhang, R., Lv, M., Wang, Y., Tan, K., Xia, X., Wan, J., Jing, M., Zhang, X., Li, Y., Yang, Y., Wang, L., Chu, J., Li, Y., & Li, Y.*. (2023).
Local 5-HT signaling bi-directionally regulates the coincidence time window for associative learning.
Neuron. 111(7): 1118-1135. e5.
[Full Text]
[PDF]
[Supplemental Data] See also BioRxiv https://doi.org/10.1101/2022.03.27.485970 |
|
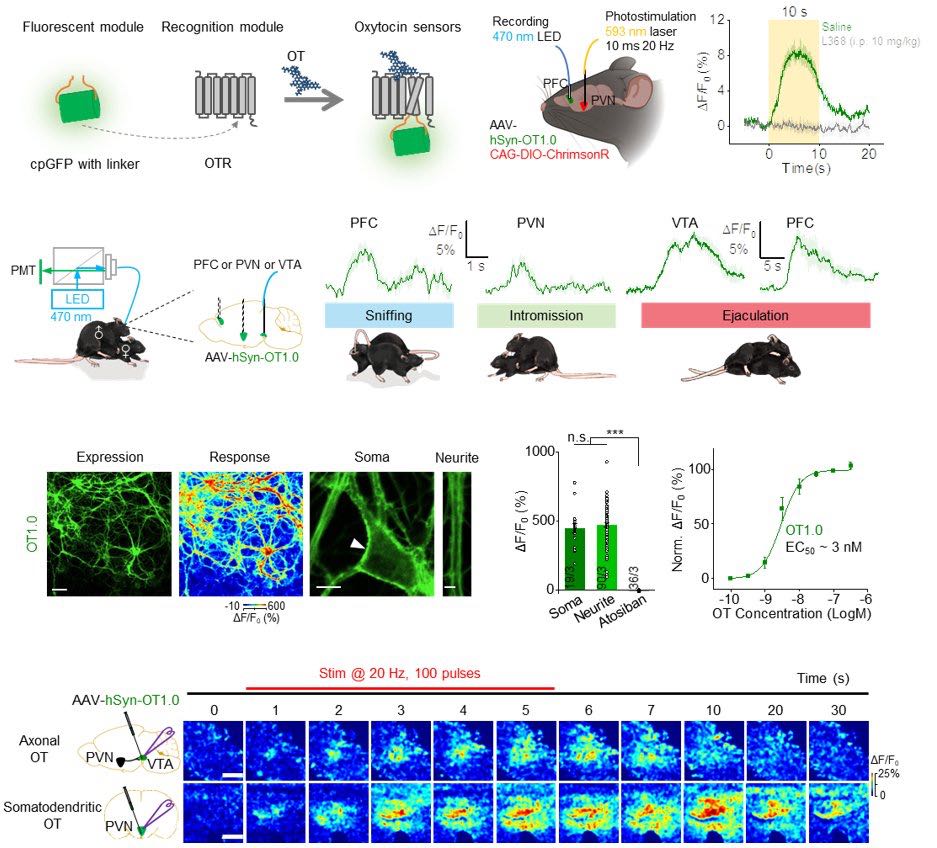 |
· Qian, T.#, Wang, H.#, Wang, P.#, Geng, L., Mei, L., Osakada, T., Wang, L., Tang, Y., Kania, A., Grinevich, V., Stoop, R., Lin, D., Luo, M., & Li, Y.* (2023).
A genetically encoded sensor measures temporal oxytocin release from different neuronal compartments.
Nature Biotechnology. 41(7): 944-957.
[Full Text]
[PDF]
[Supplemental Data]
[OT Signals in the VTA During Mating]
[OT Signals in the PVN During Mating]
[OT Signals in the PFC During Mating] See also BioRxiv https://doi.org/10.1101/2022.02.10.480016 |
|
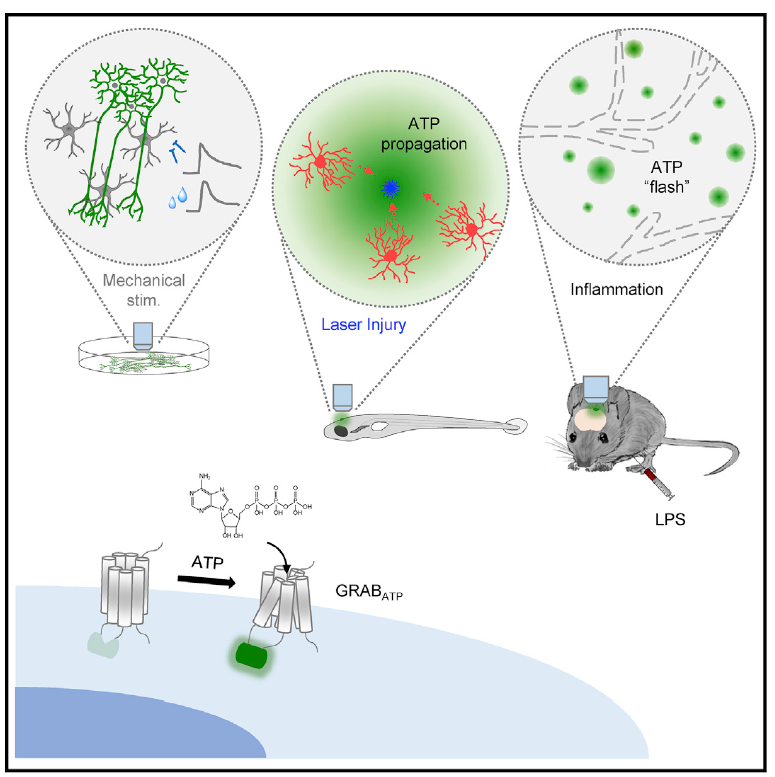 |
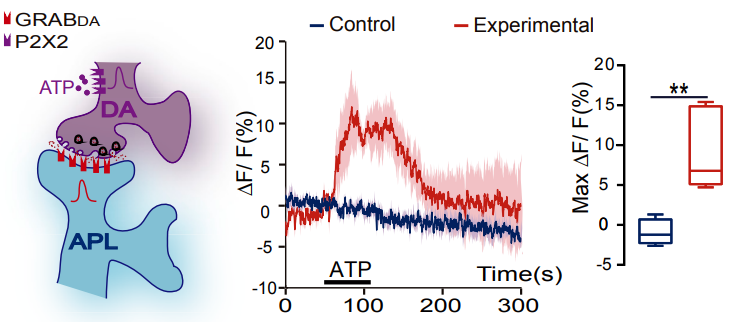
· Wu, Z.*, He, K., Chen, Y., Li, H., Pan, S., Li, B., Liu, T.,
Wang, H., Du, J., Jing, M., & Li, Y.* (2022). A sensitive GRAB sensor for detecting extracellular
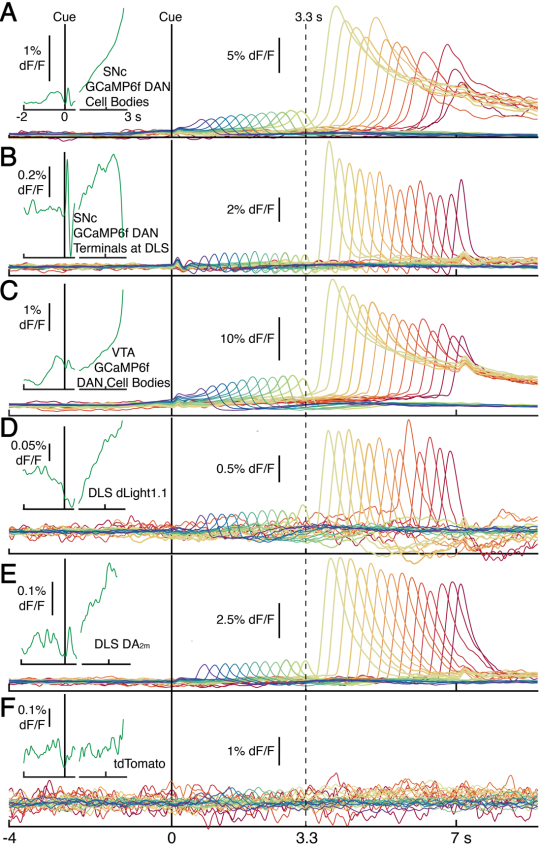
ATP in vitro and in vivo Neuron, 110(5), 770-782.e775.
[Full Text]
[PDF]
[Supplemental Data] * See Comments Highlight by: Umpierre, A. D., Haruwaka, K., & Wu, L.-J.* (2022).
Getting a sense of ATP in real time.
Neuroscience Bulletin.
[Full Text]
[PDF]
[ATP and Microglia Dynamics in Zebrafish]
[ATP Release During Inflammation in Mice] See also BioRxiv https://doi.org/10.1101/2021.02.24.432680 |
|
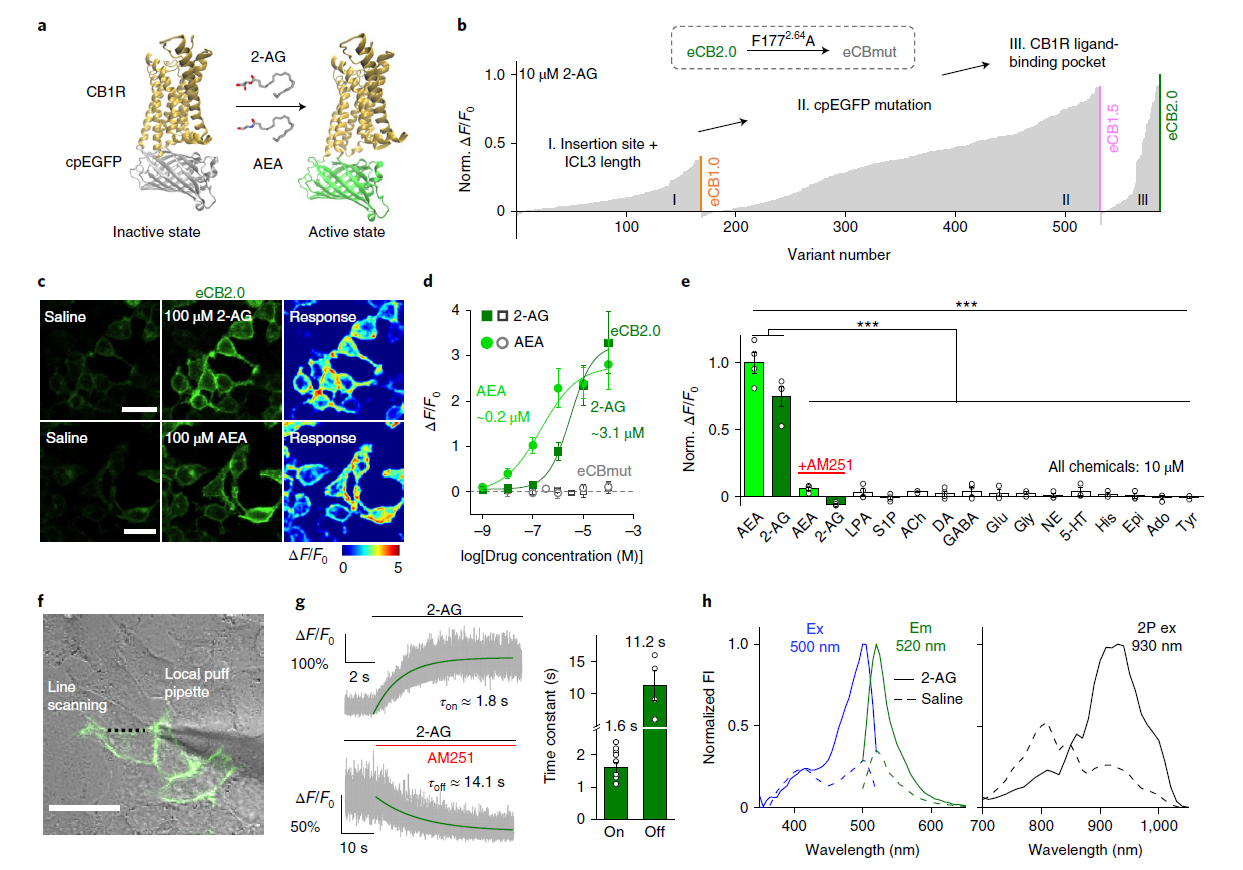 |
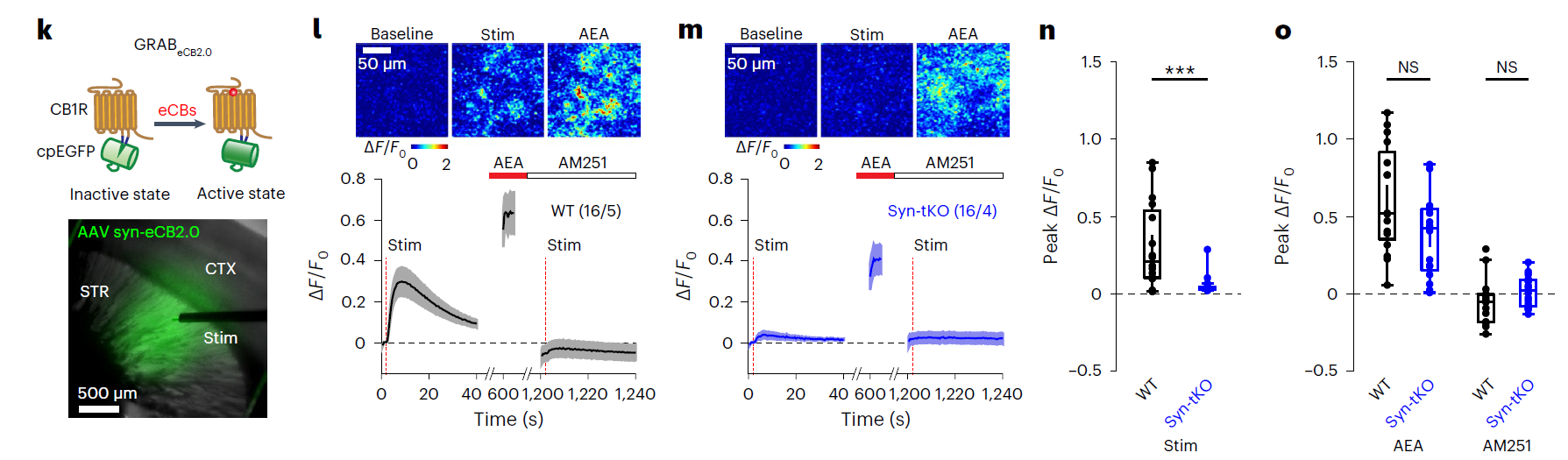
· Dong, A., He, K., Dudok, B., Farrell, J. S.,
Guan, W., Liput, D. J., Puhl, H. L., Cai, R., Wang, H., Duan, J., Albarran, E.,
Ding, J., Lovinger, D. M., Li, B., Soltesz, I., & Li, Y.*. (2021).
A fluorescent sensor for spatiotemporally resolved imaging of endocannabinoid dynamics in vivo.
Nature Biotechnology. 40(5): 787-798.
[Full Text]
[PDF]
[Supplemental Data]
[eCB and Ca2+ Signals During Seizures] See also BioRxiv https://www.biorxiv.org/content/10.1101/2020.10.08.329169 |
|
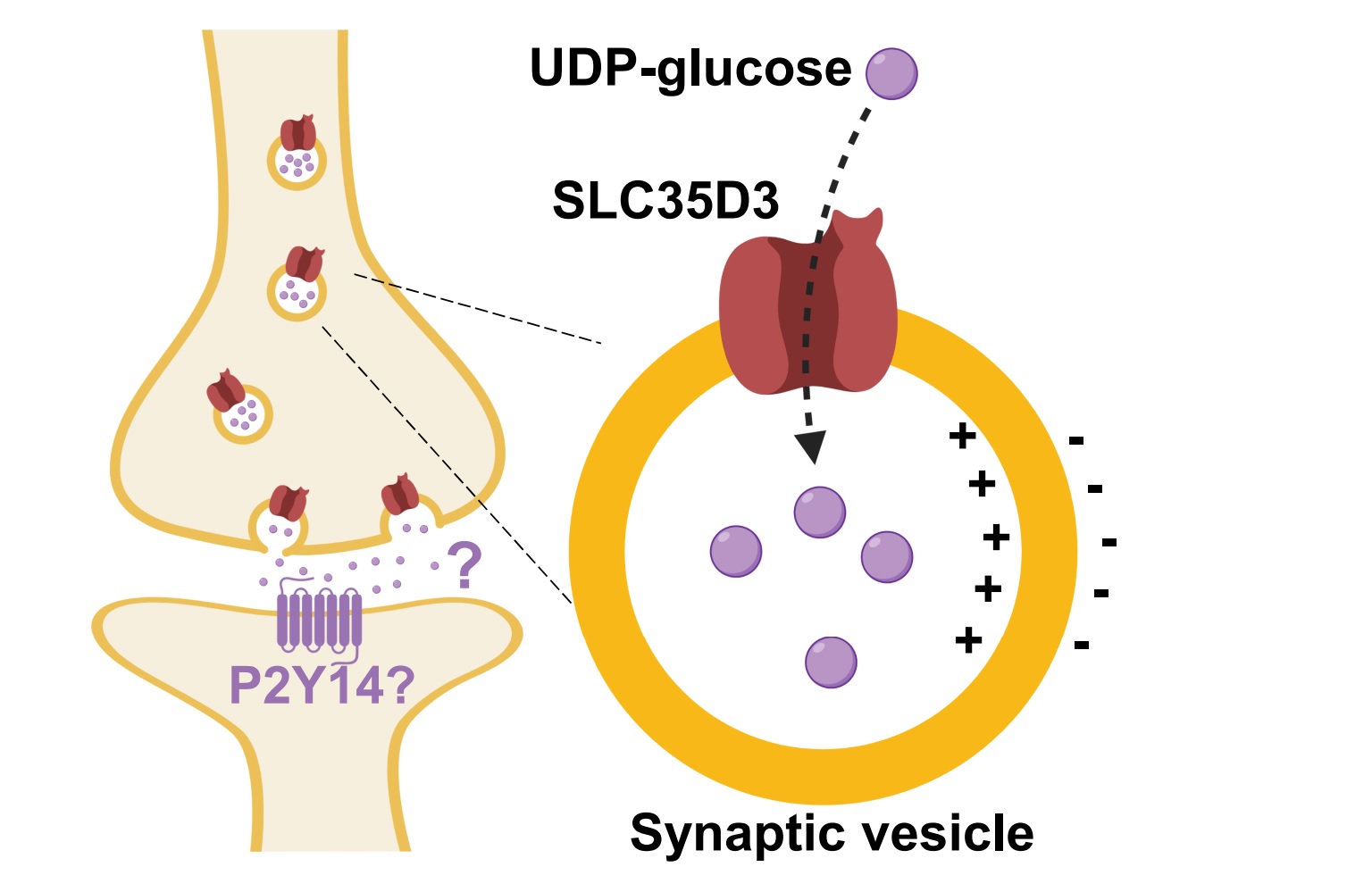 |
· Qian, C., Wu, Z., Sun, R., Yu, H., Zeng, J., Rao, Y., & Li, Y. *.
(2021). Localization, proteomics, and metabolite profiling reveal a putative vesicular transporter for UDP-glucose. eLife. 10: e65417.
[Full Text]
[PDF]
[Supplemental Data] See also BioRxiv https://doi.org/10.1101/2020.12.01.405605 |
|
 |
· Wan, J., Peng, W., Li, X., Qian, T.,
Song, K., Zeng, J., Deng, F., Hao, S., Feng,J., Zhang, P., Zhang, Y., Zou, J.,
Pan, S., Shin, M., Venton, B. J., Zhu, J. J., Jing, M., Xu, M., Li, Y.*.
(2021). A genetically encoded sensor for measuring serotonin dynamics.
Nature Neuroscience. 24(5): 746-752. https://doi.org/10.1038/s41593-021-00823-7.
[Full Text]
[PDF]
[Supplemental Data]
[5-HT Release in Drosophila] See also BioRxiv https://doi.org/10.1101/2020.02.24.962282 |
|
 |
· Sun, F.#, Zhou, J.#, Dai, B.#, Qian, T., Zeng, J., Li, X., Zhuo, Y., Zhang, Y., Wang, Y., Qian, C., Tan, K., Feng, J., Dong, H., Lin, D.*, Cui, G.*, & Li, Y.*.(2020).
Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nature Methods. 17(11), 1156-1166.
[Full Text]
[PDF]
[Supplemental Data]
[Mushroom Body DA Dynamics] |
|
 |
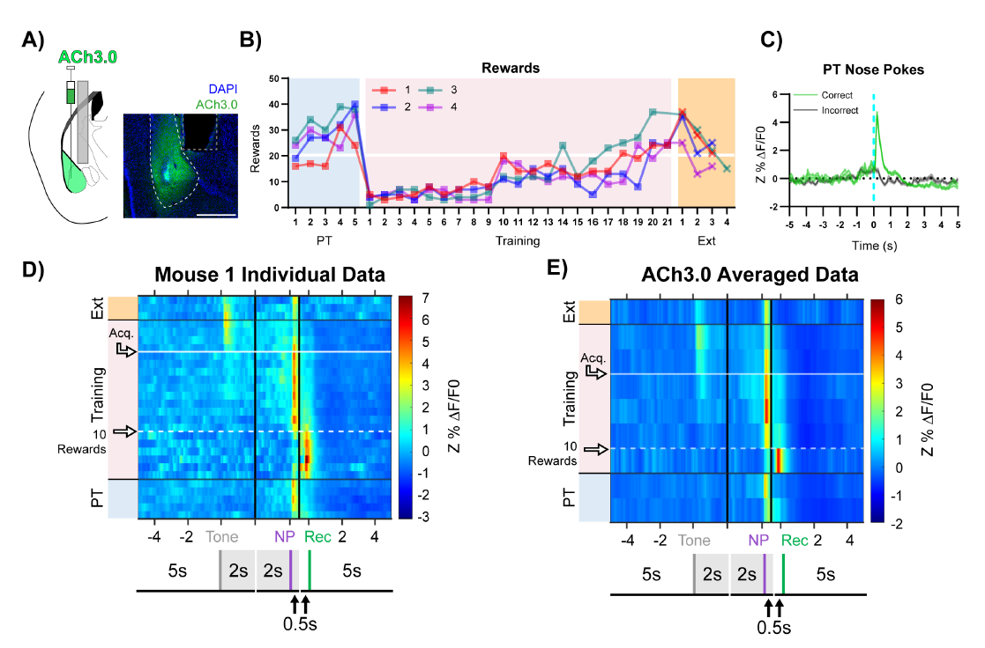
· Jing, M.*, Li, Y., Zeng, J., Huang, P., Skirzewski, M., Kljakic, O., Peng, W., Qian, T., Tan, K., Zou, J. , Trinh, S., Wu, R., Zhang, S., Pan, S., Hires, S., Xu, M., Li, H., Saksida, L. M., Prado, V. F., Bussey, T., Prado, M. A. M., Chen, L., Cheng, H., Li, Y.*.(2020).
An optimized acetylcholine sensor for monitoring in vivo cholinergic activity. Nature Methods, 17(11), 1139-1146.
[Full Text]
[PDF]
[Supplemental Data] |
|
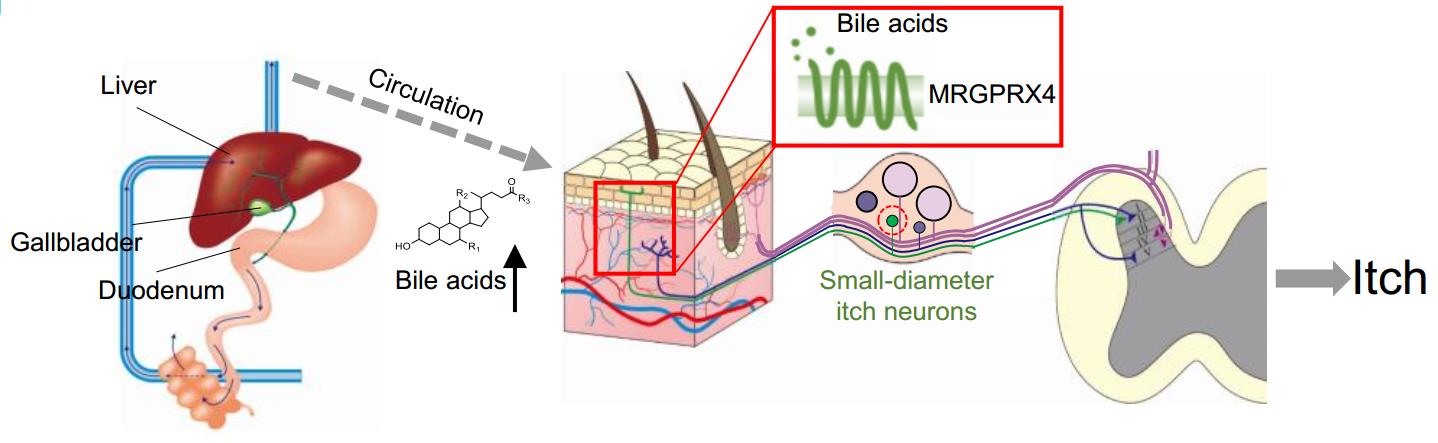 |
· Yu, H., Zhao, T., Liu, S., Wu, Q., Johnson, O., Wu, Z., Zhuang, Z., Shi, Y., He, R., Yang, Y., Sun, J., Wang, X., Xu, H., Zeng, Z., Lei, X., Luo, W.* & Li, Y.*.
(2019). MRGPRX4 is a bile acid receptor for human cholestatic itch. eLife, 8, e48431.
[Full Text]
[PDF]
[Supplemental Data] |
|
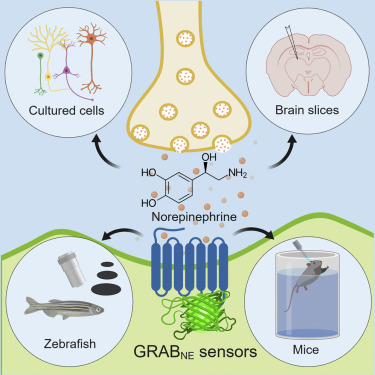 |
· Feng, J., Zhang, C., Lischinsky, J. E., Jing, M., Zhou, J., Wang, H., Zhang, Y., Dong, A., Wu, Z., Wu, H., Chen, W., Zhang, P., Zou, J., Hires, S. A., Zhu, J. J., Cui, G., Lin, D., Du, J. & Li, Y.* (2019). A genetically encoded fluorescent sensor for rapid and specific in vivo detection of norepinephrine. Neuron, 102(4), 745-761.
[Full Text]
[PDF]
[Supplemental Data]
[Video Abstract] |
|
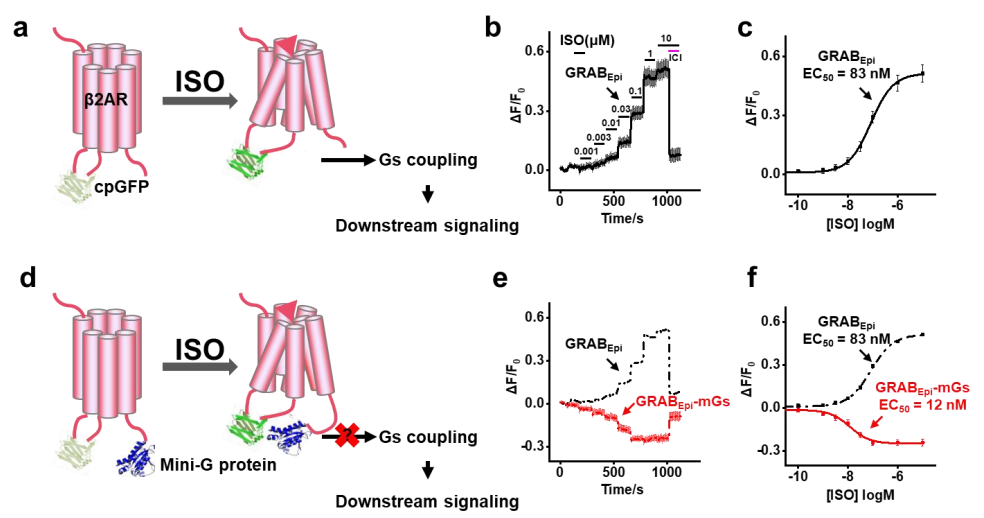 |
· Wu, Z.#, Feng, J.#, Jing, M., & Li, Y.* (2019). G protein-assisted optimization of GPCR-activation based (GRAB) sensors.
Neural Imaging and Sensing 2019, vol. 10865, p. 108650N. International Society for Optics and Photonics.
[Full Text]
[PDF] |
|
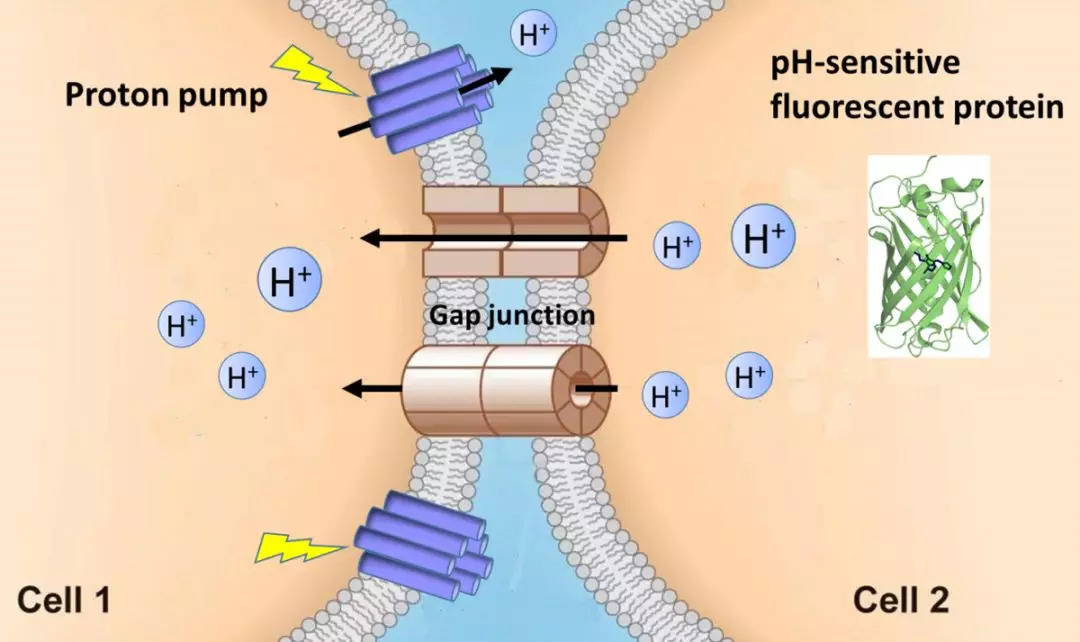 |
· Wu, L., Dong, A., Dong, L., Wang, S. Q., & Li, Y*. (2019). PARIS, an optogenetic method for functionally mapping gap junctions. eLife, 8, e43366.
[Full Text]
[PDF]
[Supplemental Data] * See Insight by: Kick, D. R., & Schulz, D. J. (2019). Cell Communication: Studying gap junctions with PARIS. eLife, 8, e45207. [Full Text][PDF] |
|
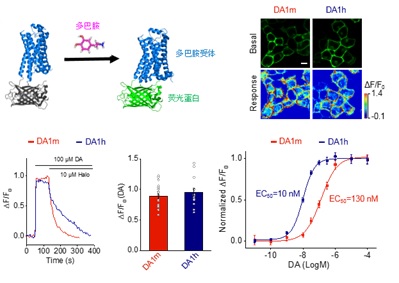 |
· Sun, F.#, Zeng, J.#, Jing, M.#, Zhou, J.,
Feng, J., Owen, S., Luo, Y., Li, F., Wang, H., Yamaguchi, T., Yong, Z.,
Gao, Y., Peng, W., Wang, L., Zhang, S., Du, J., Lin, D., Xu, M., Kreitzer, A. C., Cui, G.
& Li, Y.* (2018). A genetically-encoded fluorescent sensor enables rapid and
specific detection of dopamine in flies, fish, and mice. Cell,
174(2), 481-496.
[Full Text]
[PDF]
[Supplemental Data]
[DA Signals Under Electrical Stimulation]
[DA Signals Under Odor Stimulation] * See Viewpoint by: Beyene, A. G., Delevich, K., Yang, S. J., & Landry, M. P. (2018). New optical probes bring dopamine to light. Biochemistry, 6379-6381. [Full Text][PDF> |
|
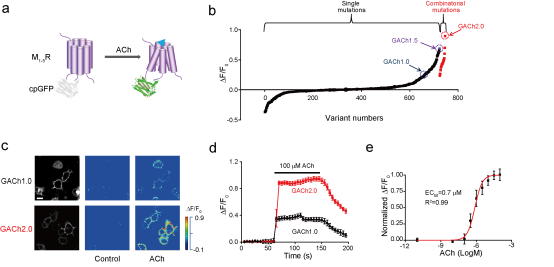 |
· Jing, M.#, Zhang, P.#, Wang, G., Feng, J., Mesik, L., Zeng, J., Jiang, H., Wang, S., Looby, J. C., Guagliardo, N. A., Langma, L. W., Lu, J., Zuo, Y., Talmage, D. A., Role, L. W., Barrett, P. Q., Zhang, L. I., Luo, M., Song, Y., Zhu, JJ* & Li, Y*.
(2018). A genetically-encoded fluorescent acetylcholine indicator for in vitro and in vivo studies. Nature Biotechnology, 36(8), 726-737.
[Full Text]
[PDF][Supplemental Data]
[Supplemental Videos] * See Research Highlight by: Vogt, N. (2018). Detecting acetylcholine. Nature methods, 15(9), 648. [Full Text] [PDF] |
|
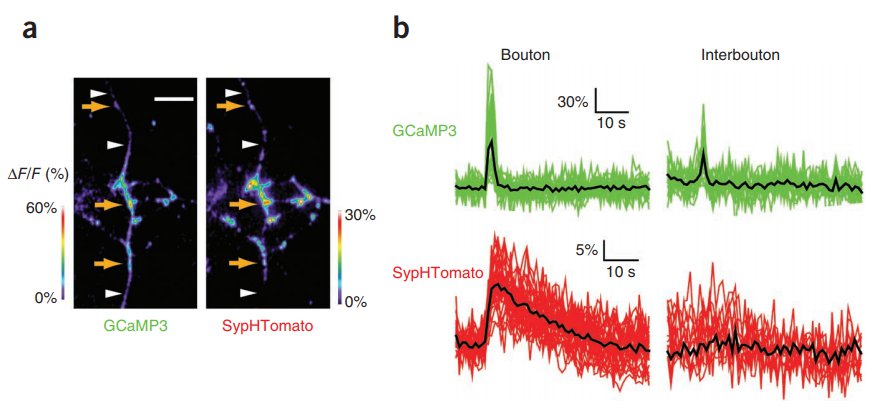 |
· Li, Y.*, & Tsien, R. W.* (2012). pHTomato, a red, genetically encoded indicator that enables multiplex interrogation of synaptic activity. Nature neuroscience, 15(7), 1047-1053.
[Full Text]
[PDF]
[Supplemental Data] |
|
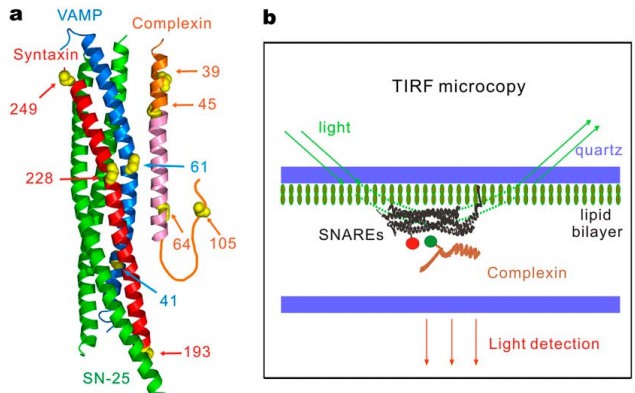 |
· Li, Y., Augustine, G. J., & Weninger, K.* (2007). Kinetics of complexin binding to the SNARE complex: correcting single molecule FRET measurements for hidden events. Biophysical journal, 93(6), 2178-2187.
[Full Text]
[PDF]
[Supplemental Data] |
综述,书评等
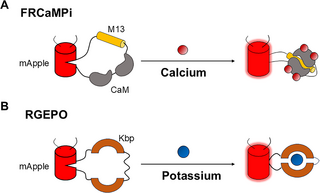 |
· Geng, L., & Li, Y.* (2025).
Illuminating calcium and potassium dynamics with red fluorescent sensors.
PLOS Biology.
[Full Text]
[PDF] |
|
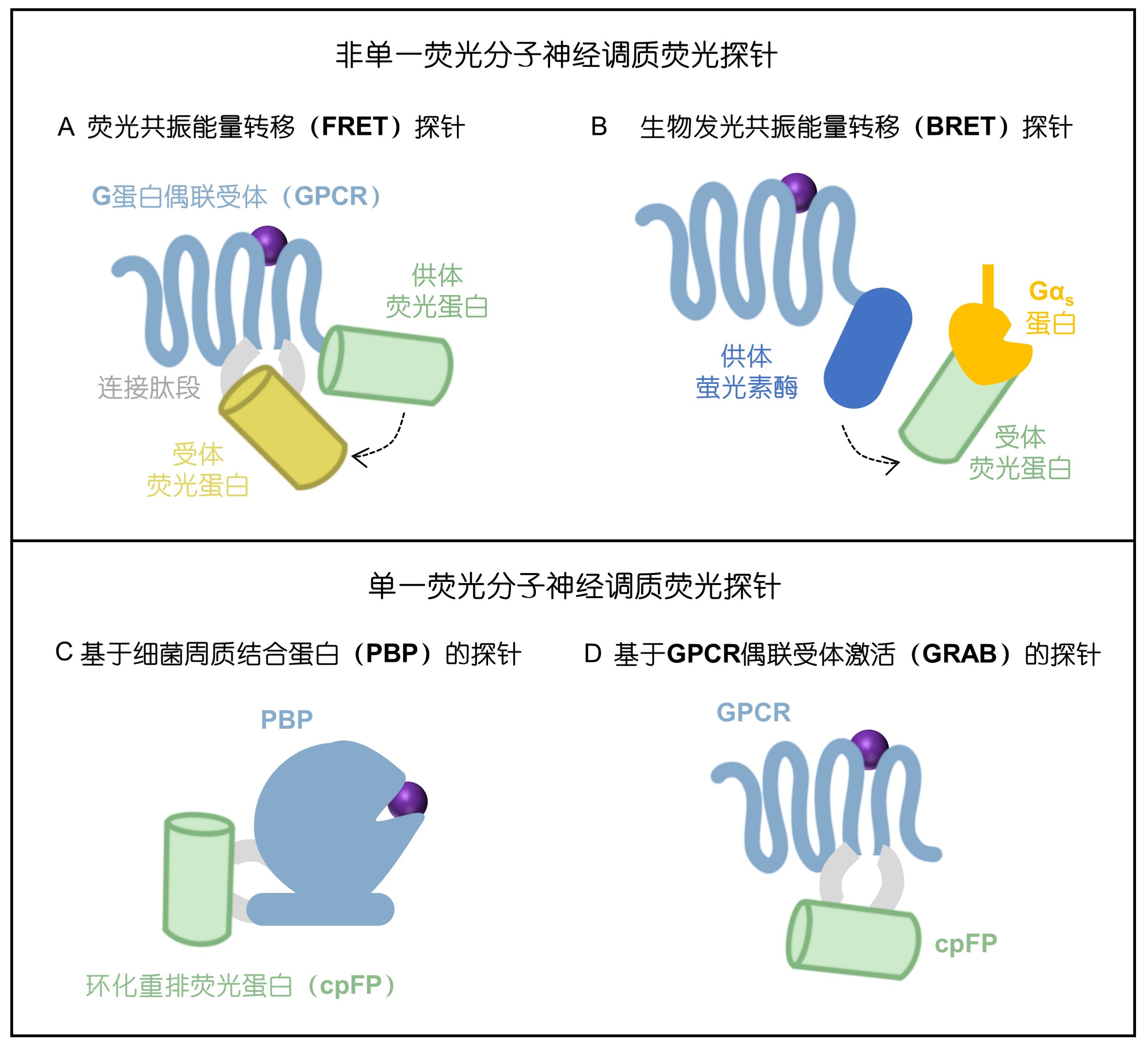 |
· Feng, J., & Li, Y.* (2025).
Research Progress and Prospects of Genetically Encoded Neuromodulator Sensors.
SCIENTIA SINICA Vitae. 2025, ISSN 1674-7232. (In Chinese)
[Full Text]
[PDF] |
|
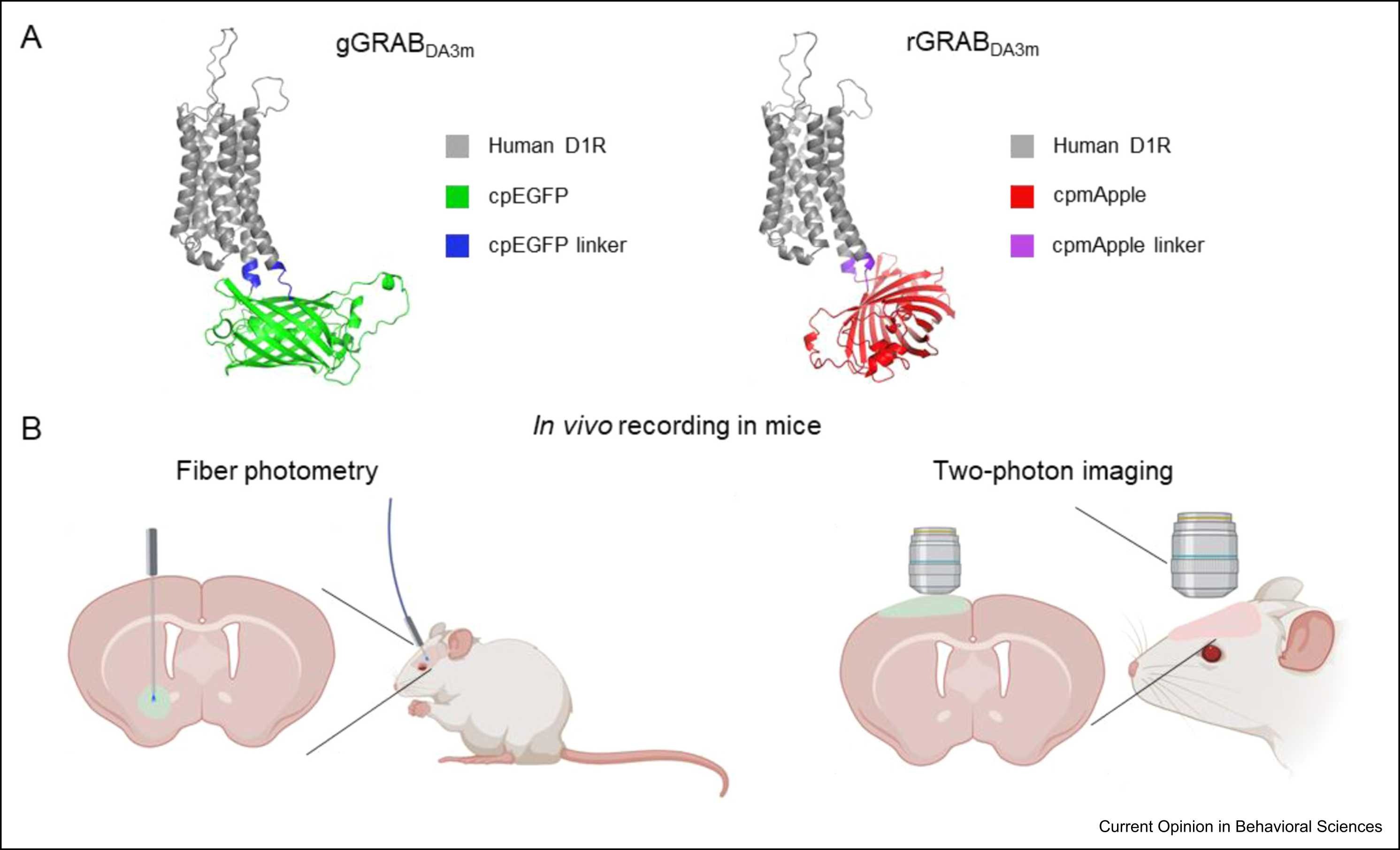 |
· Dong, H., Wang, Z., & Li, Y.* (2025).
Genetically encoded dopamine sensors: principles, applications, and future directions.
Current Opinion in Behavioral Sciences. Volume 62, April 2025, 101489.
[Full Text]
[PDF] |
|
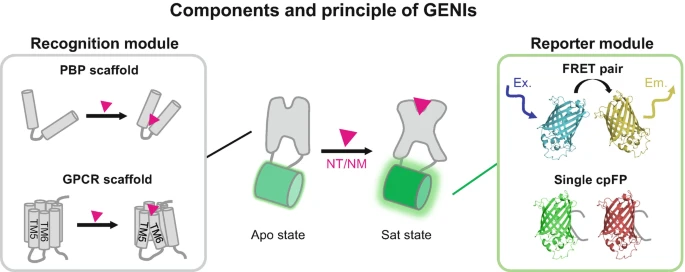 |
· Deng, F., Feng, J., Xie,H., & Li, Y.* (2025).
Mesoscopic Imaging of Neurotransmitters and Neuromodulators with Genetically Encoded Sensors.
Awake Behaving Mesoscopic Brain Imaging. Neuromethods, vol 214. Humana, New York.
[Full Text]
[PDF] |
|
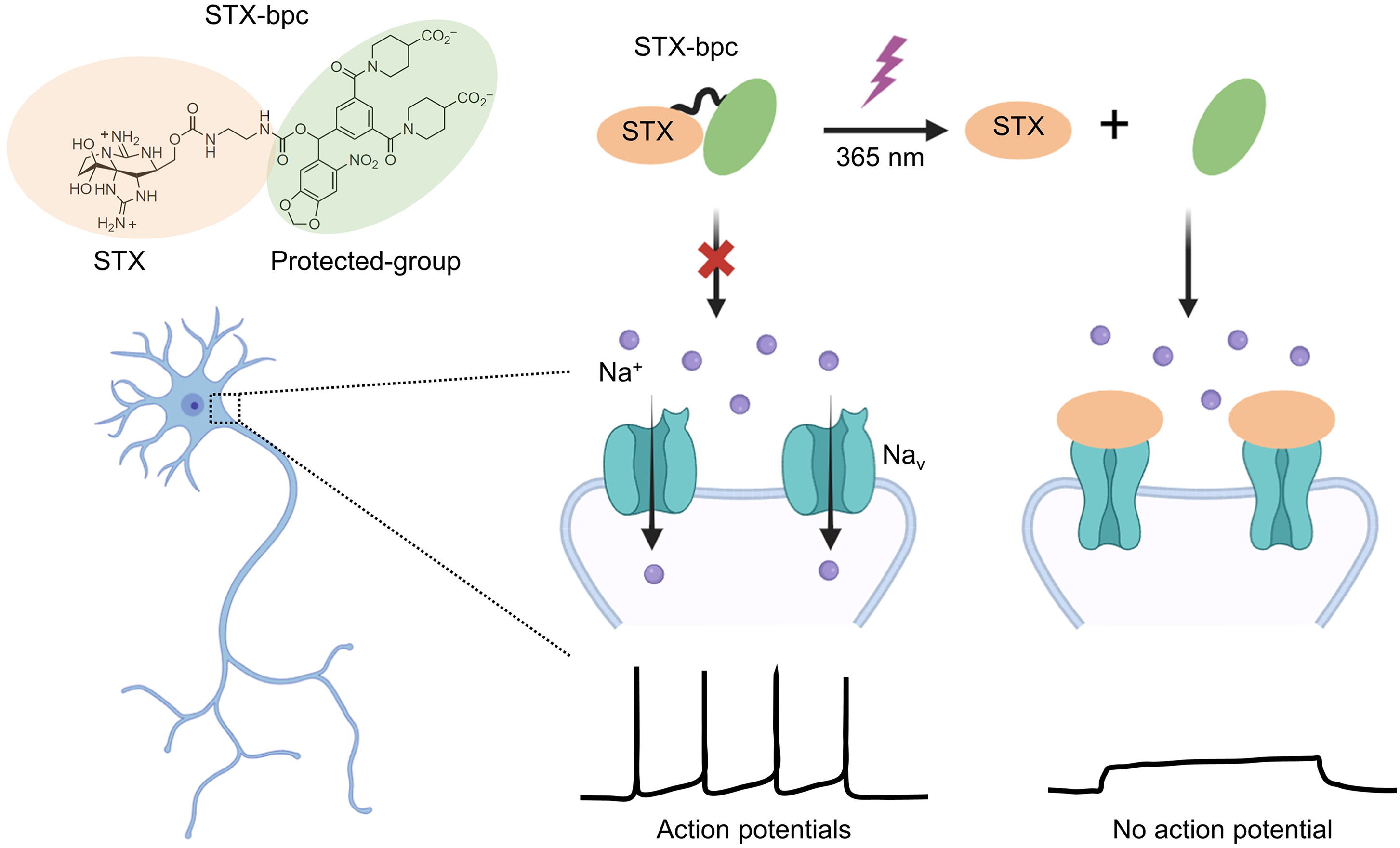 |
· Wan, J., & Li, Y.* (2024).
STX-bpc: “Brightening” the path to neuronal inhibition.
Cell Chemical Biology. 31(7): 1233-1235.
[Full Text]
[PDF] |
|
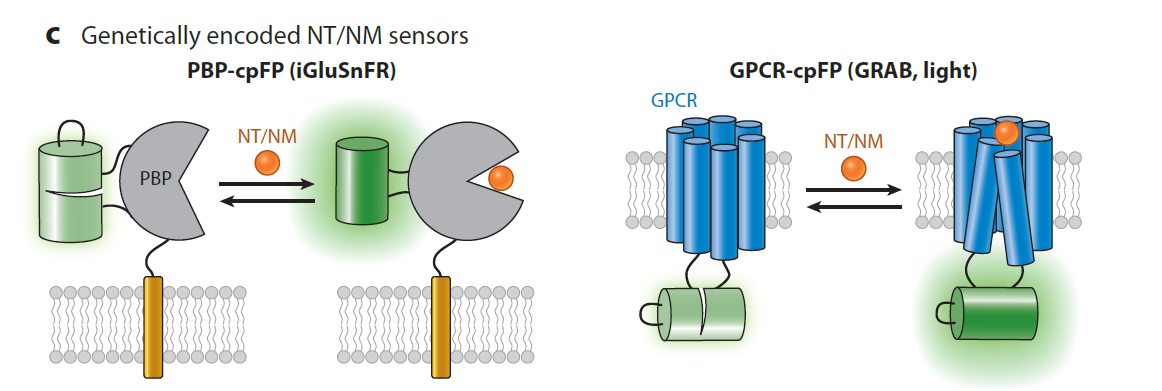 |
· Yang, Y.#, Li, B.#, & Li, Y.* (2024).
Genetically Encoded Sensors for the In Vivo Detection of Neurochemical Dynamics.
Annual Review of Analytical Chemistry. 17.
[Full Text]
[PDF] |
|
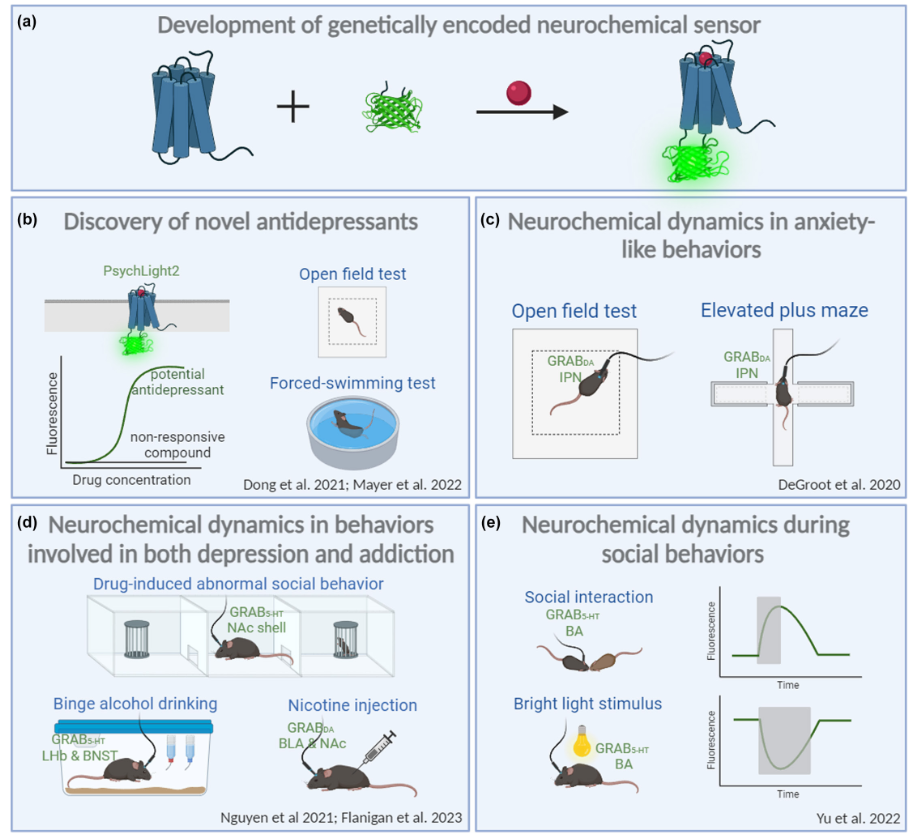 |
· Zhao, Y., Wan, J., & Li, Y.* (2024).
Genetically encoded sensors for in vivo detection of neurochemicals relevant to depression.
Journal of Neurochemistry. 17.
[Full Text]
[PDF] |
|
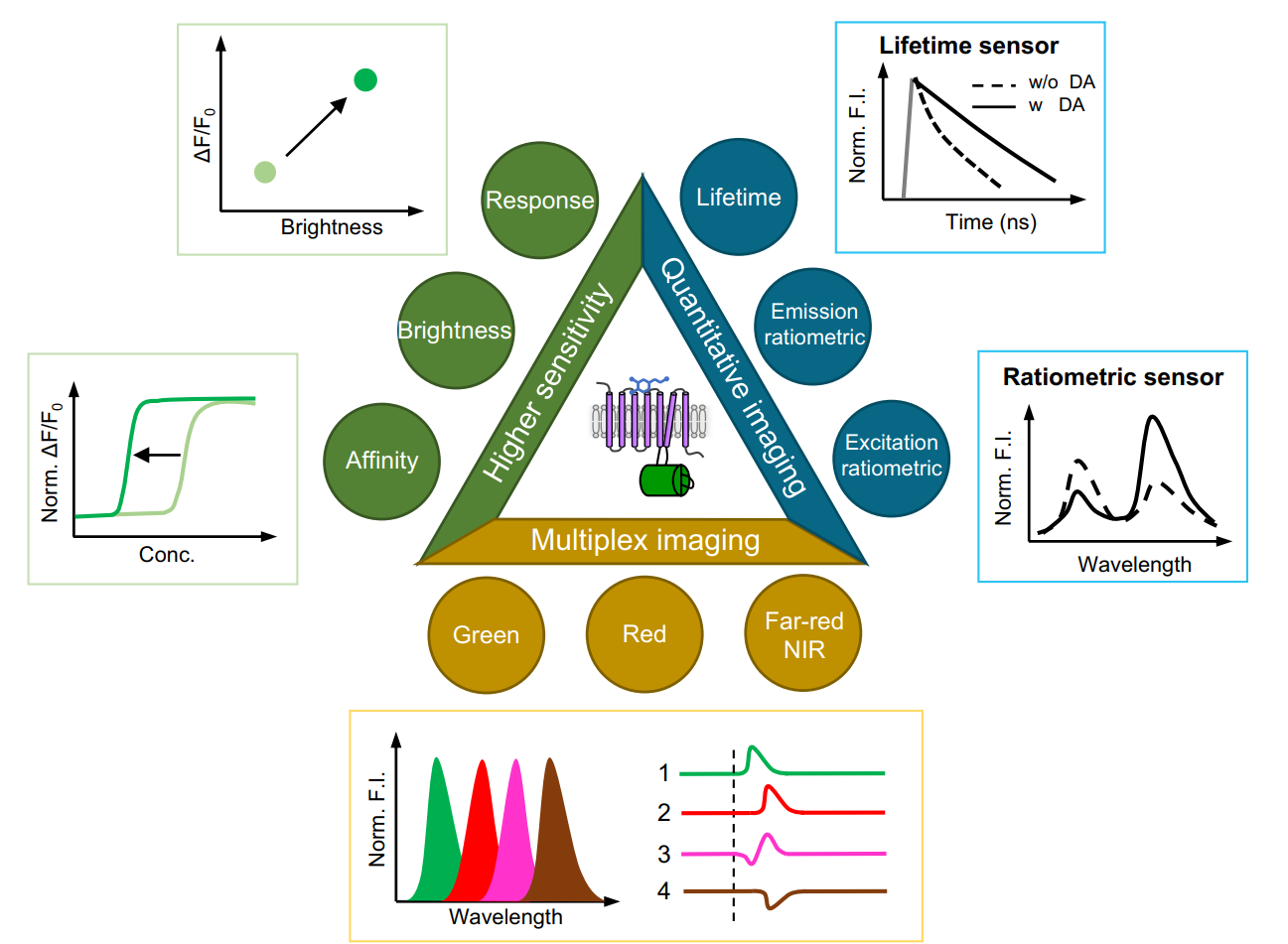 |
· Zheng, Y., & Li, Y.* (2023).
Past, Present, and Future of Tools for Dopamine Detection.
Neuroscience, 525, 13-25.
[Full Text]
[PDF] |
|
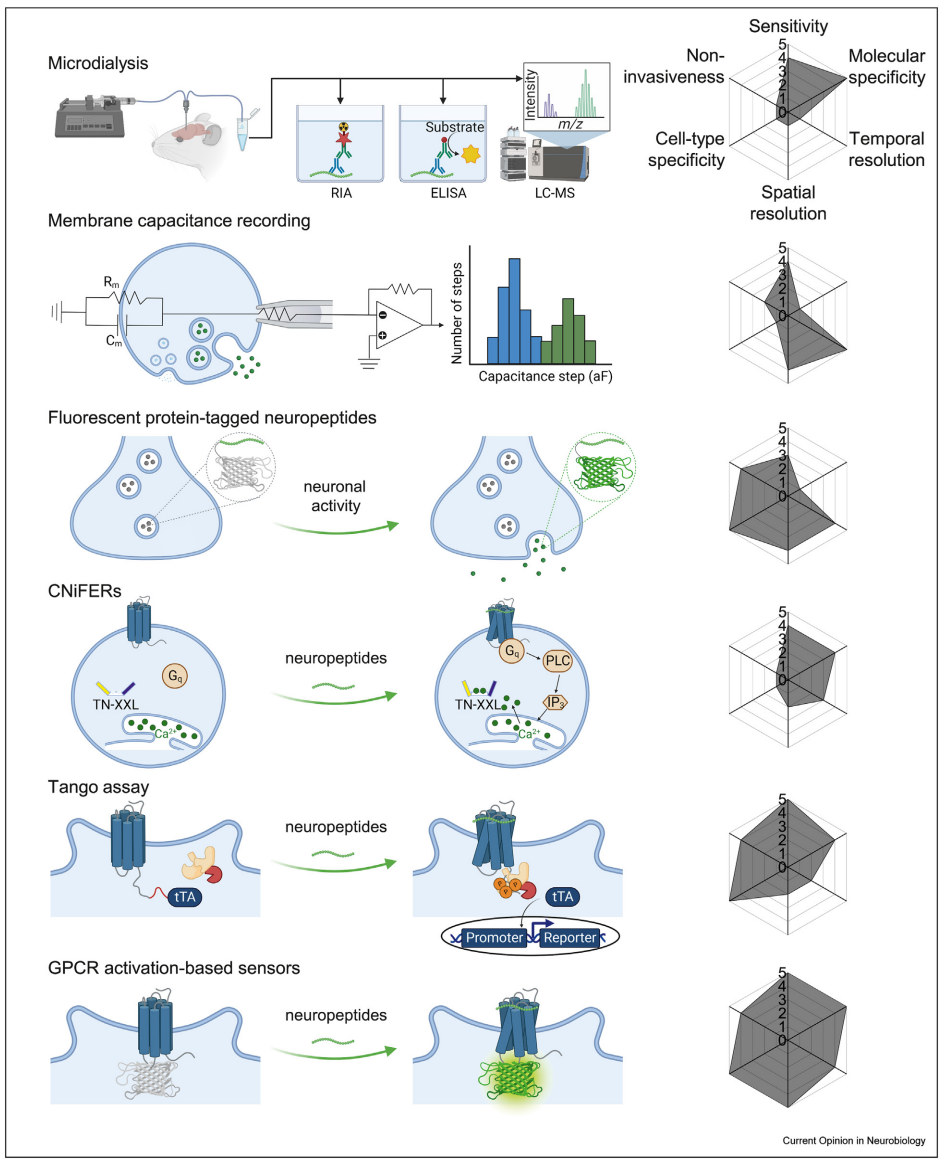 |
· Qian, T., Wang, H., Xia, X., & Li, Y.* (2023)
Current and emerging methods for probing neuropeptide transmission.
Current Opinion in Neurobiology, 81, 102751.
[Full Text]
[PDF] |
|
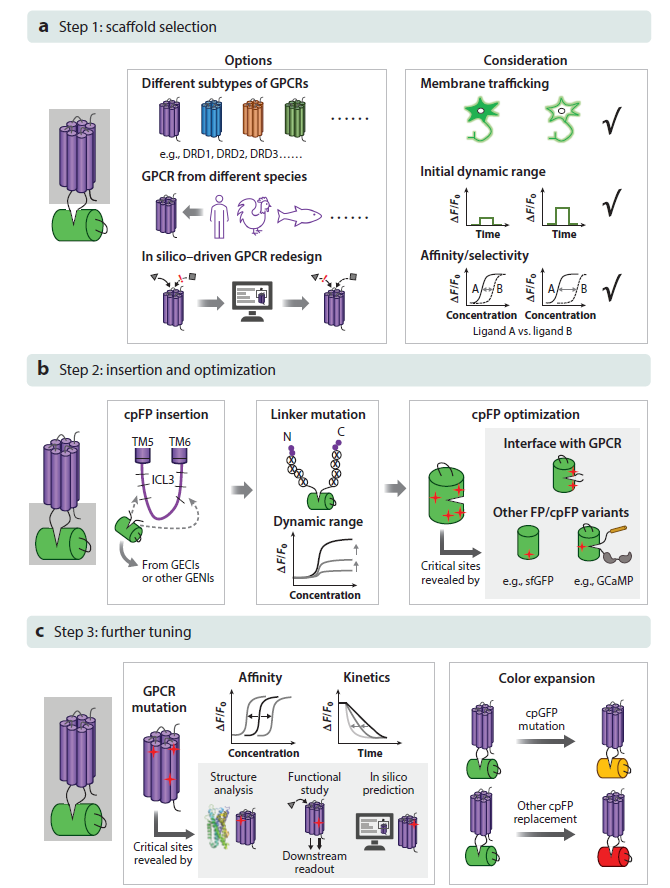 |
· Dong, C.#, Zheng, Y.#, Long-Iyer, K., Wright, E. C., Li, Y.*, & Tian, L.* (2022).
Fluorescence imaging of neural activity, neurochemical dynamics, and drug-specific receptor conformation with genetically encoded sensors.
Annual Review of Neuroscience. 45(1): 273-294.
[Full Text]
[PDF] |
|
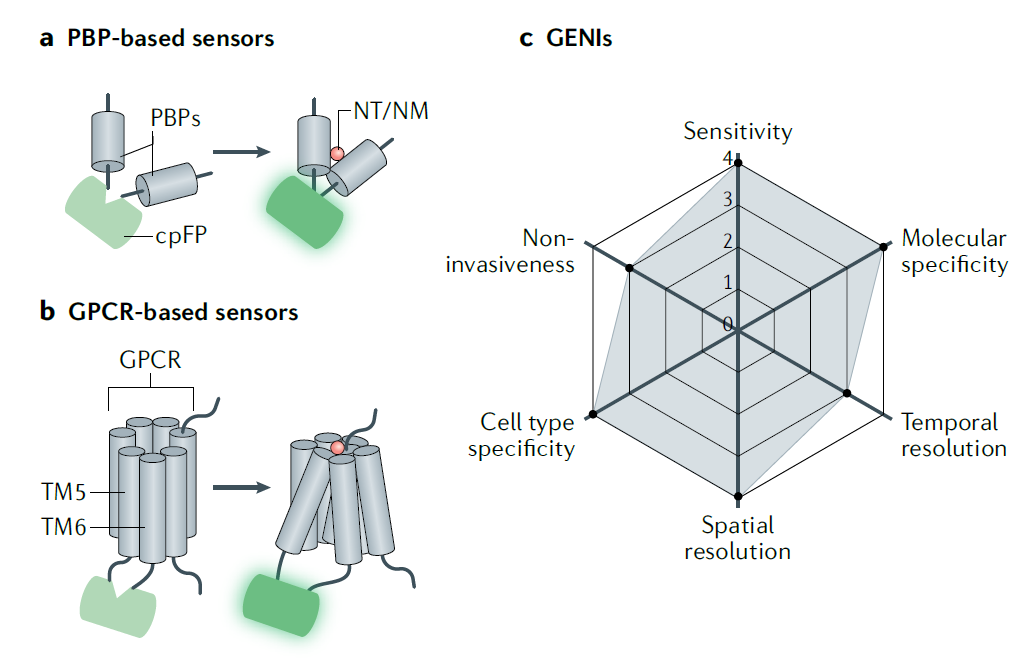 |
· Wu, Z., Lin, D., & Li, Y.* (2022).
Pushing the frontiers: tools for monitoring neurotransmitters and neuromodulators.
Nature Reviews Neuroscience. 23(5): 257-274.
[Full Text]
[PDF] |
|
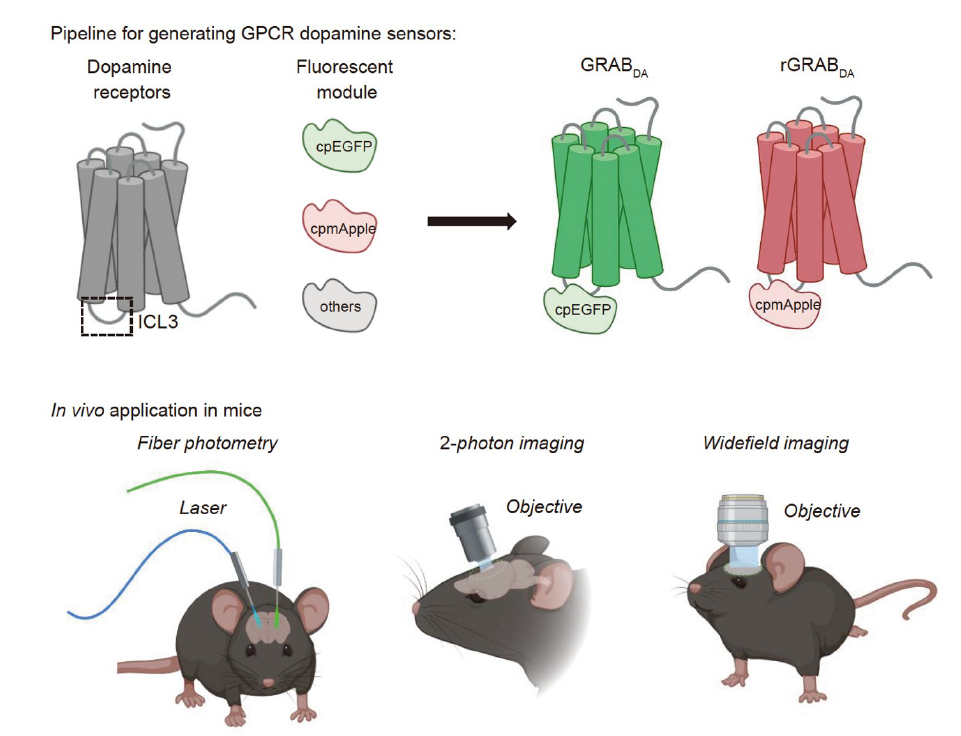 |
· Zhuo, Y., Li, Y.* (2022). New imaging methods for monitoring dopaminergic neurotransmission. Science China Life Sciences, 65.
[Full Text]
[PDF] |
|
 |
· Yulong Li. (2021). Neuron, 109(21), 3346-3348.
[Full Text]
[PDF] |
|
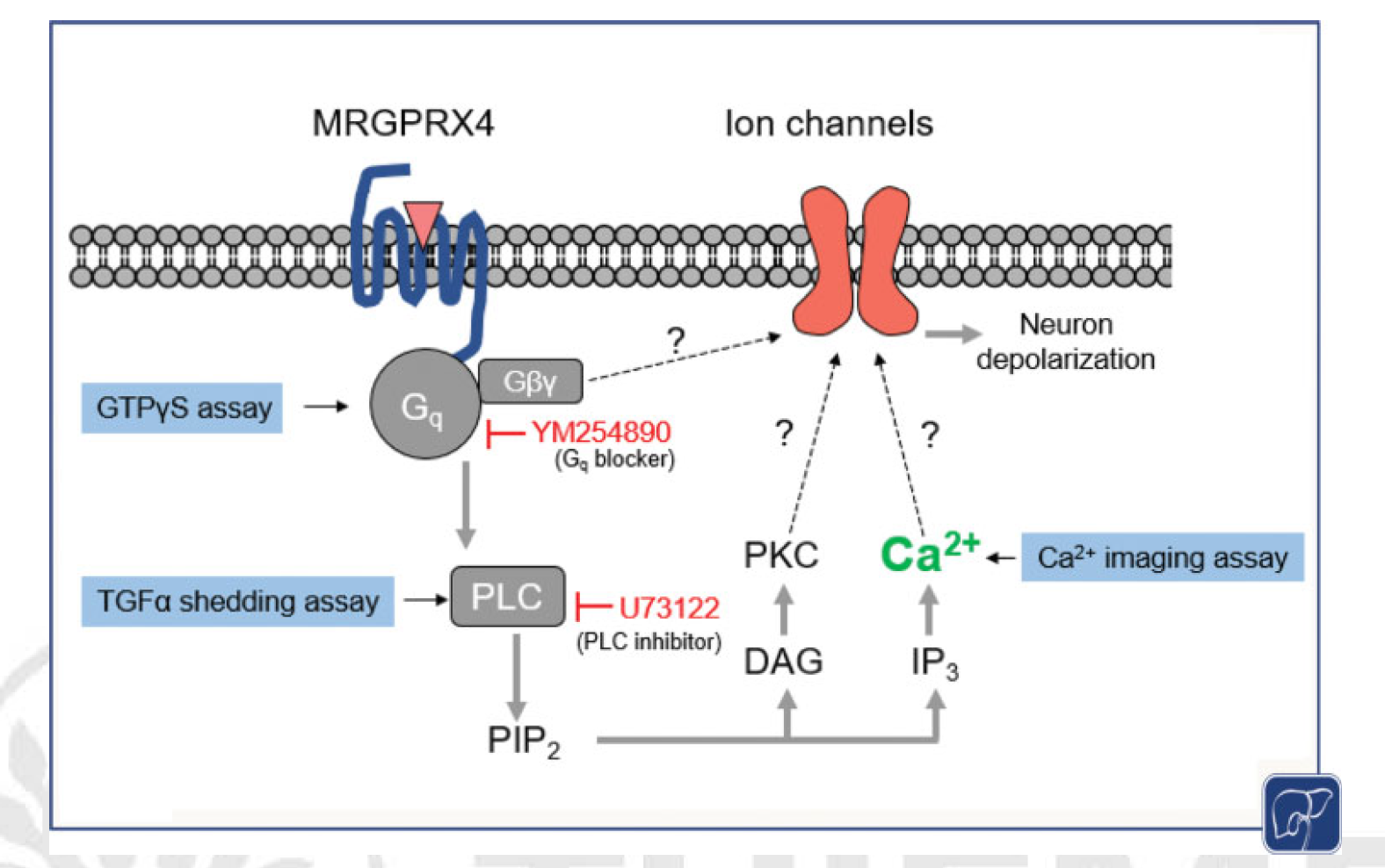 |
· Yu, H., Wangensteen, K., Deng, T., Li, Y., & Luo, W.* (2021). MRGPRX4 in Cholestatic Pruritus. Semin Liver Dis41(03), 358-367.
[Full Text]
[PDF] |
|
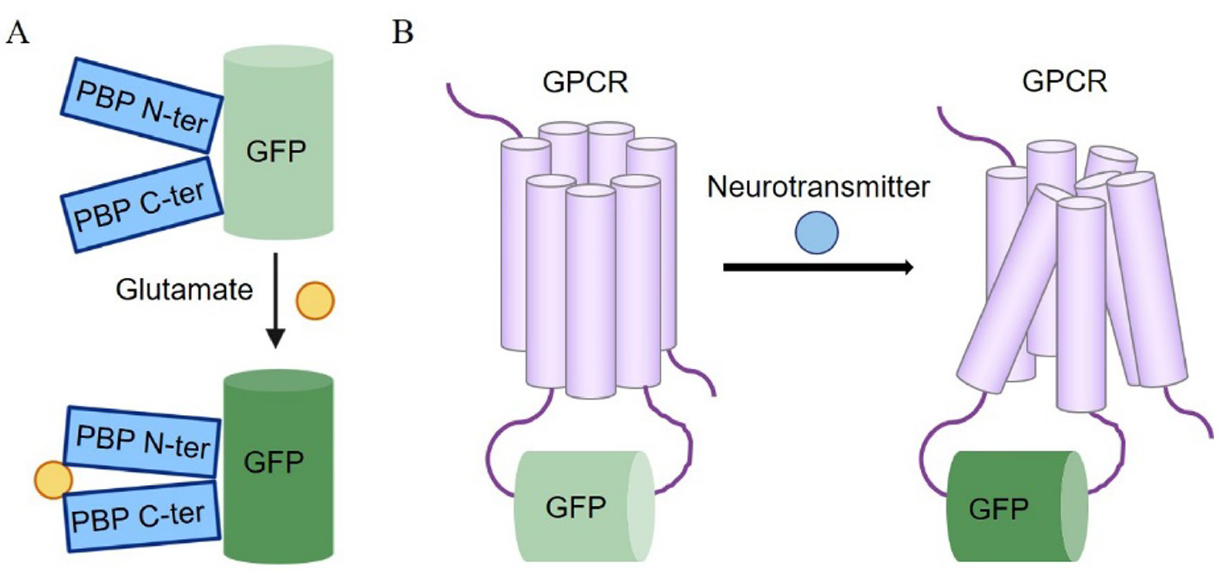 |
· Wan, J. & Li, Y.* (2020). Recent advances in detection methods for neurotransmitters. Chinese Journal of Analytical Chemistry, 48(3), 307-315. (In Chinese)
[Full Text]
[PDF] |
|
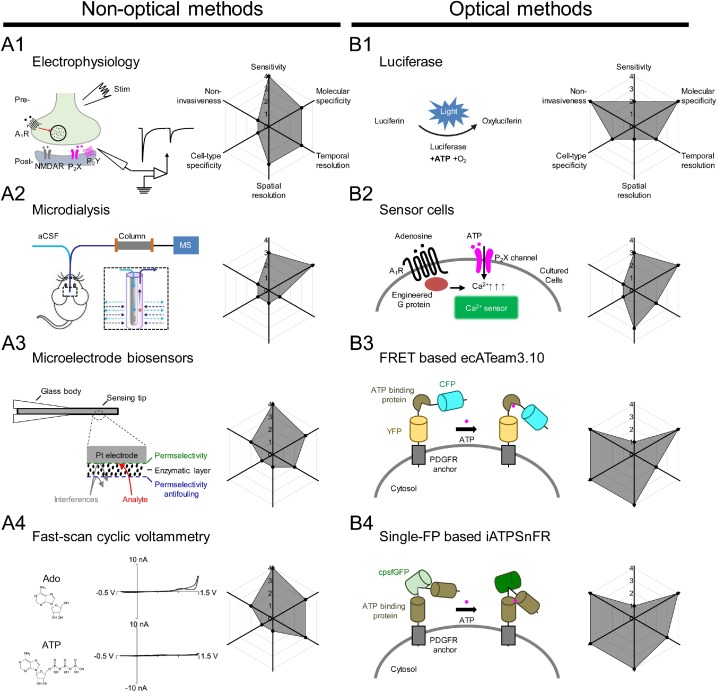 |
· Wu, Z.* & Li, Y.* (2020). New frontiers in probing the dynamics of purinergic transmitters in vivo. Neuroscience Research.
[Full Text]
[PDF] |
|
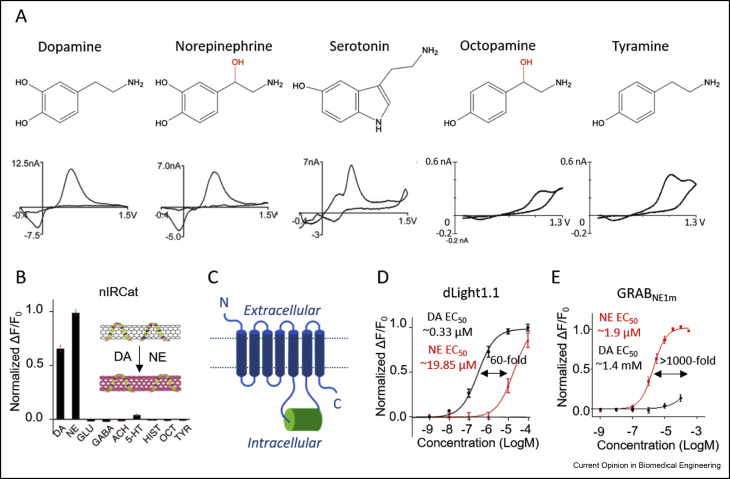 |
· Zeng, J., Sun, F., Wan, J., Feng, J. & Li, Y.* (2019). New optical methods for detecting monoamine neuromodulators. Current Opinion in Biomedical Engineering, https://doi.org/10.1016/j.cobme.2019.09.010.
[Full Text]
[PDF] |
|
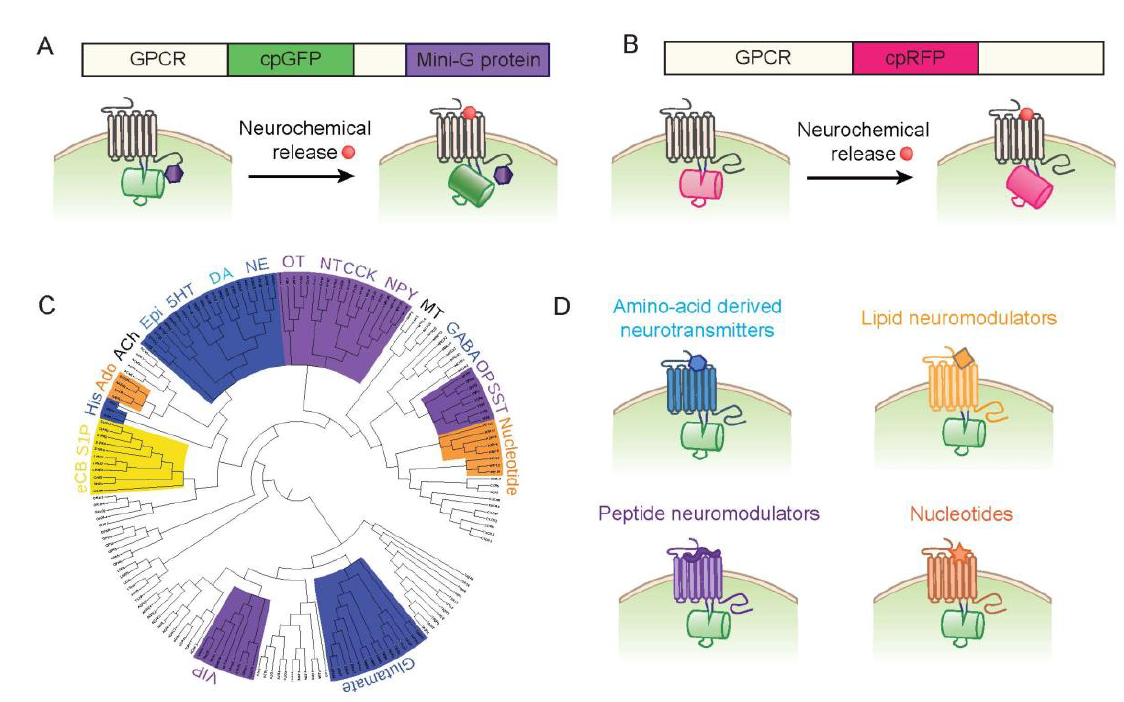 |
· Jing, M., Zhang, Y., Wang, H. & Li, Y.* (2019). GPCR‐based sensors for imaging neurochemicals with high sensitivity and specificity. Journal of Neurochemistry. [Full Text] [PDF] |
|
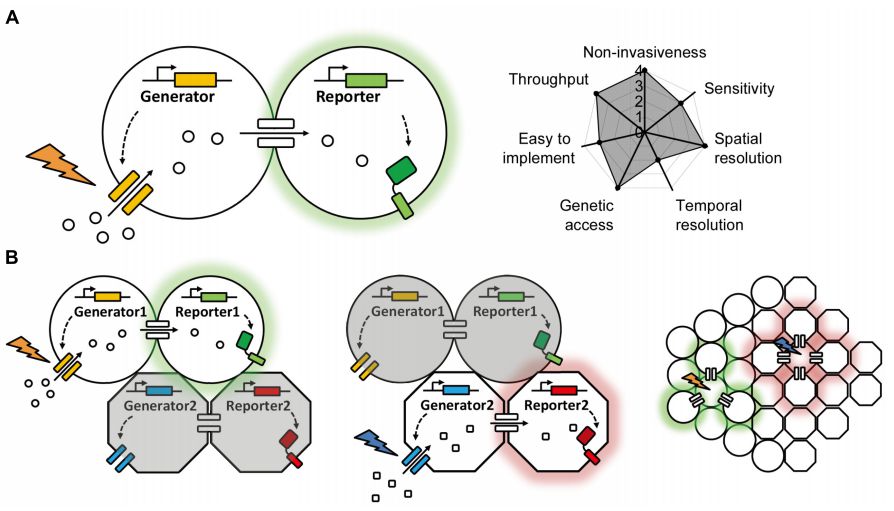 |
· Dong, A.*, Liu, S., & Li, Y.* (2018). Gap junctions in the nervous system: probing functional connections using new imaging approaches. Frontiers in Cellular Neuroscience, 12, 320.
[Full Text]
[PDF] |
|
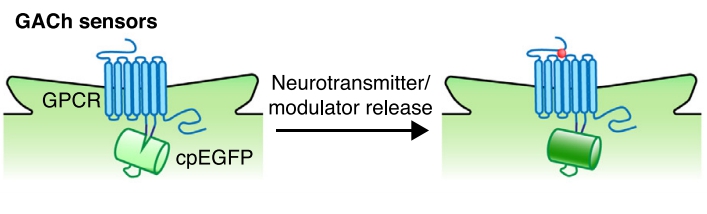 |
· Wang, H., Jing, M., & Li, Y.* (2018). Lighting up the brain: genetically encoded fluorescent sensors for imaging neurotransmitters and neuromodulators. Current Opinion in Neurobiology, 50, 171-178.
[Full Text]
[PDF] |
|
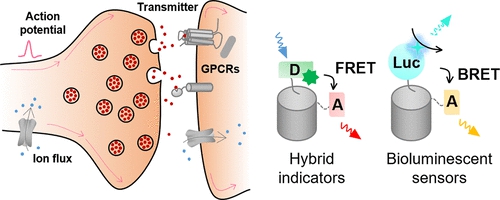 |
· Wang, A.#, Feng, J.#, Li, Y.*, & Zou, P.* (2018). Beyond fluorescent proteins: hybrid and bioluminescent indicators for imaging neural activities. ACS chemical neuroscience, 9(4), 639-650.
[Full Text]
[PDF] |
|
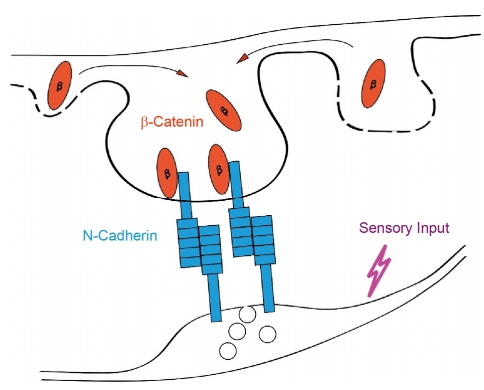 |
· Qian, C., & Li, Y.* (2015). Spine maturation and pruning during development: Cadherin/Catenin complexes come to help. Science China. Life sciences,58(9), 929.
[Full Text]
[PDF] |
|
 |
· Li, Y.*, & Rao, Y.* (2015). Pied piper of neuroscience. Cell, 163(2), 267-268.
[Full Text]
[PDF] |
预印本
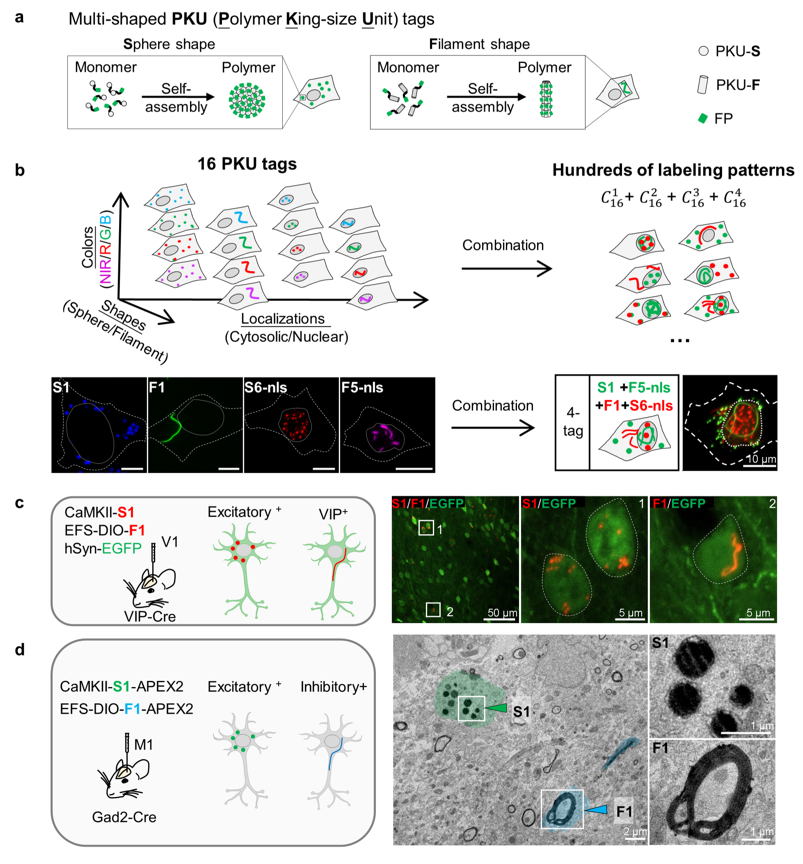 |
· Sun, R.*, Yang, P., Wang, L., Hu, Y., Yang, Y., Deng, F., Li, S., Fan, M., Xia. X., & Li, Y.* (2025).
PKU tags, novel genetically encoded shape tags for cell labeling in light and electron microscopy.
bioRxiv.
[Full Text]
[PDF] |
|
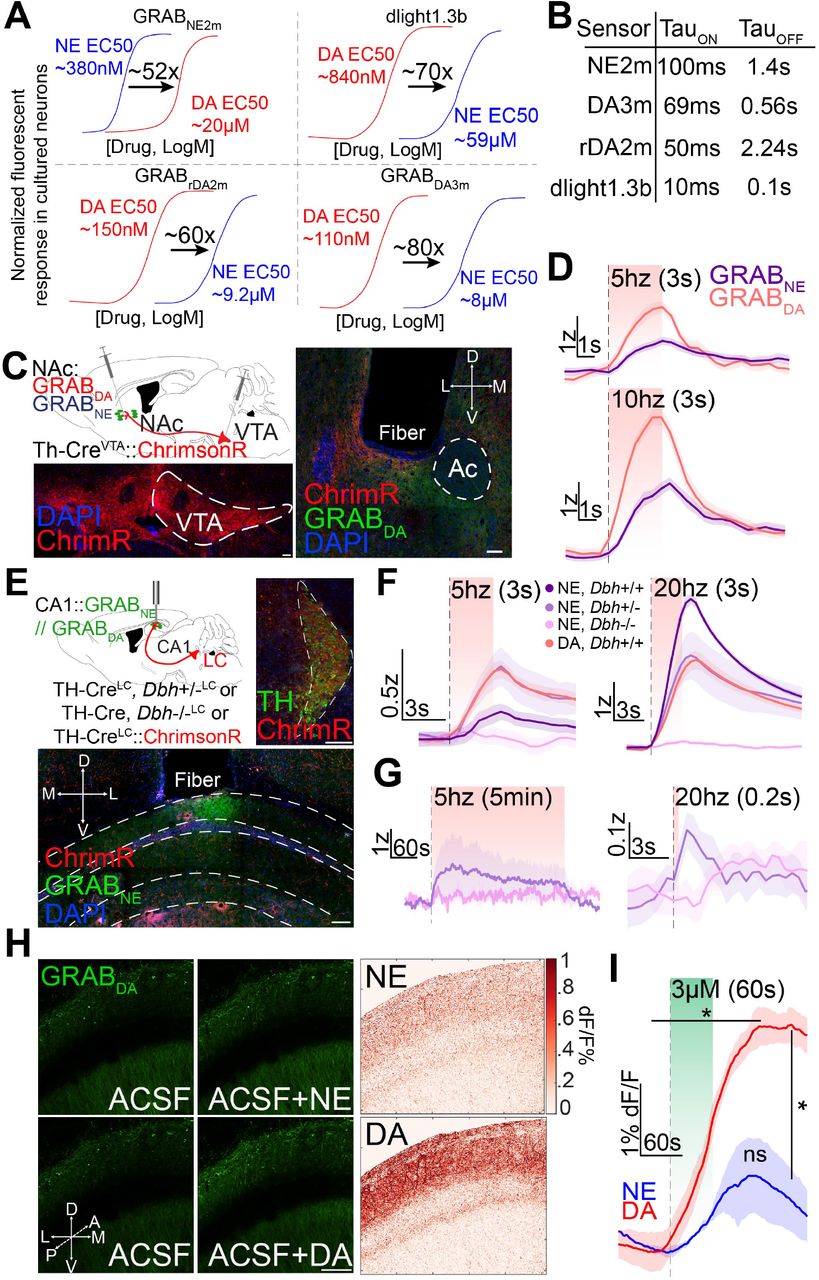 |
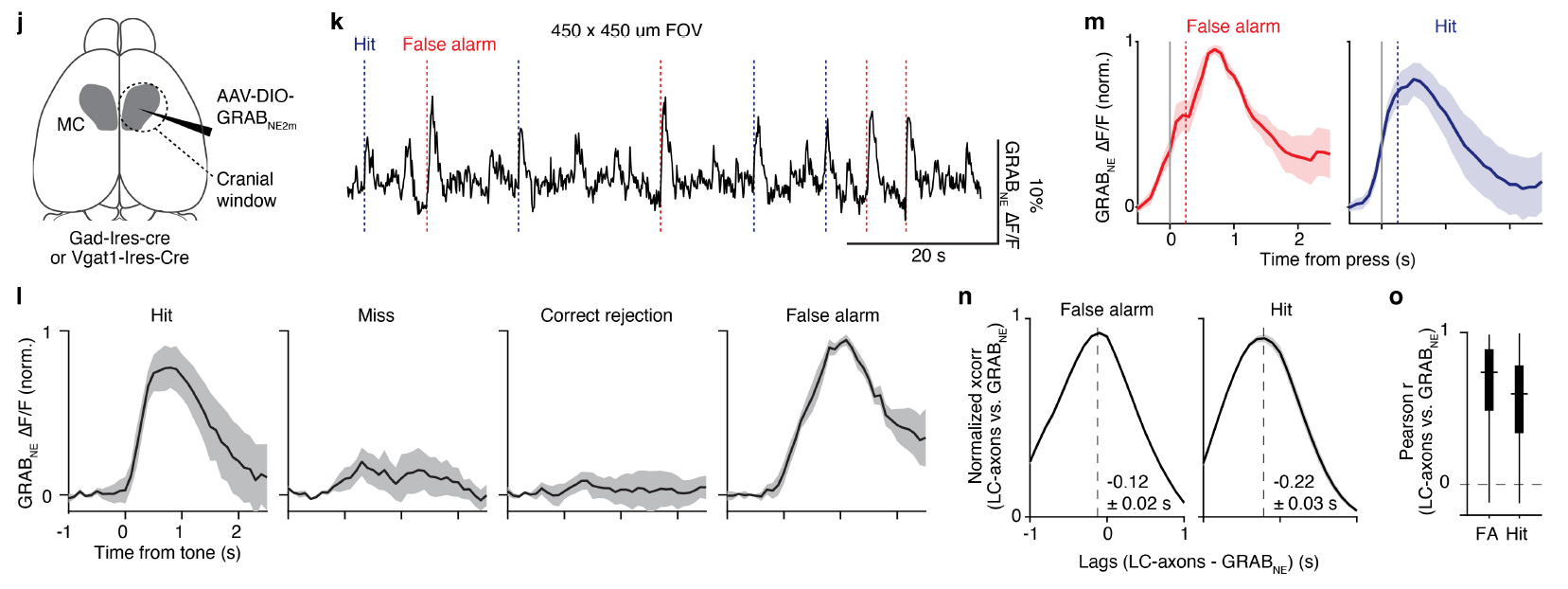
· Matarasso, A., Reyes, I., Seaholm, E., Cheeyandira, A., Piantadosi, S., Li, L., Li, Y., Weinshenkerl D., Bruchas, M.* (2025).
Stimulus-Dependent Dopamine Dynamics from Locus Coeruleus Axons.
bioRxiv.
[Full Text]
[PDF] |
|
 |
· Liu, X., Huang, H., Feng, X., Li, X., Ye, J., Yang, H., Liu, J., Liang, Z., Guo, Z., Cai, R., Cai, S., Li, Y., Wu, Z., Wang, L., Wang, F.* (2025).
A neural circuit for female-specific defensive homeostasis in risk assessment.
bioRxiv.
[Full Text]
[PDF] |
|
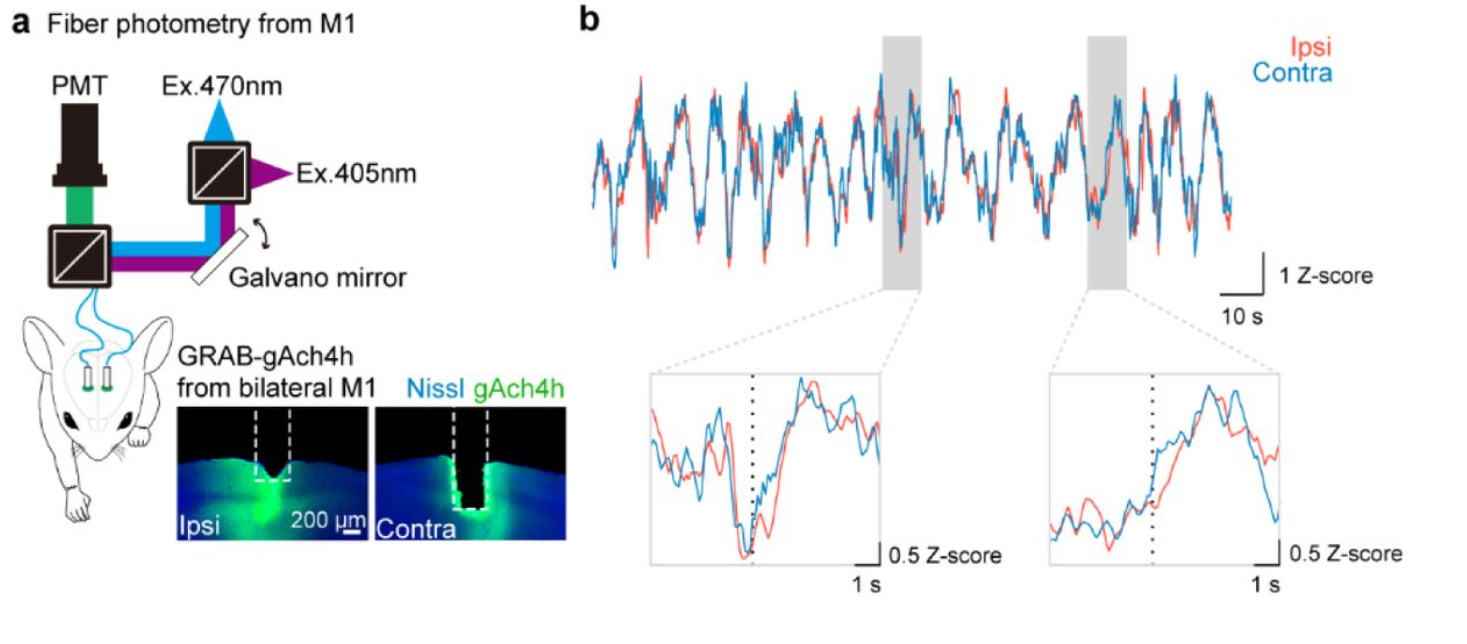 |
· Okamoto K., Tanaka, Y., Kato, S., Xie, S., Li, G., Kobayashi, K., Koike, M., Li, Y., Hioki, H.* (2025).
Paw switching with lateralized cholinergic modulation.
bioRxiv.
[Full Text]
[PDF] |
|
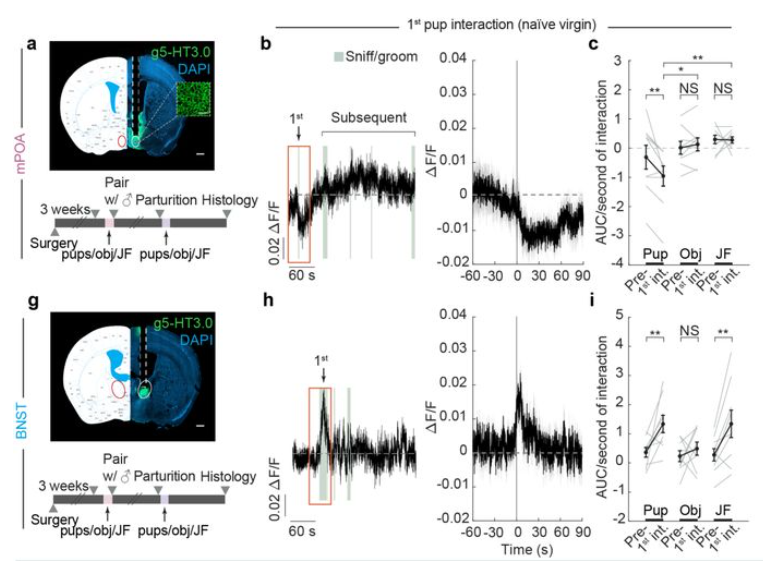 |
· Xiao, S., Chen, C., Horvath, P., Tsai, V., Cardenas, V., Biderman, D., Deng, F., Li, Y., Linderman, S., Dulac, C., & Luo, L.* (2025).
Concerted actions of distinct serotonin neurons orchestrate female pup care behavior.
bioRxiv.
[Full Text]
[PDF] |
|
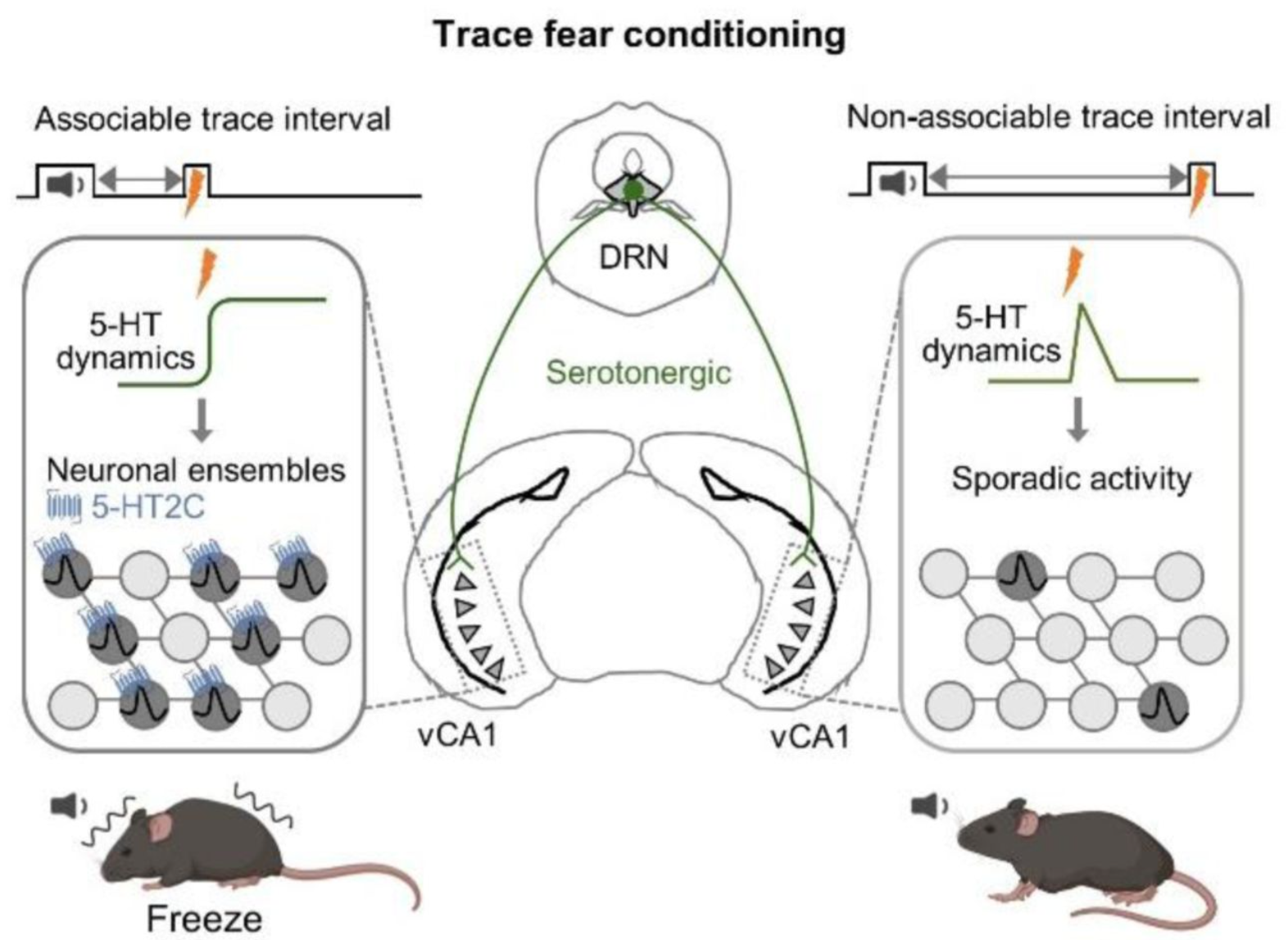 |
· Zhao, Y., Li, X., Chang, T., Xie, S., Wu, Y., Li, S., Cao, J., Wang, D., Deng, F., Feng, J., Huang, Y., & Li, Y.* (2025).
Serotonin shapes the temporal window for associative fear learning.
bioRxiv.
[Full Text]
[PDF] |
|
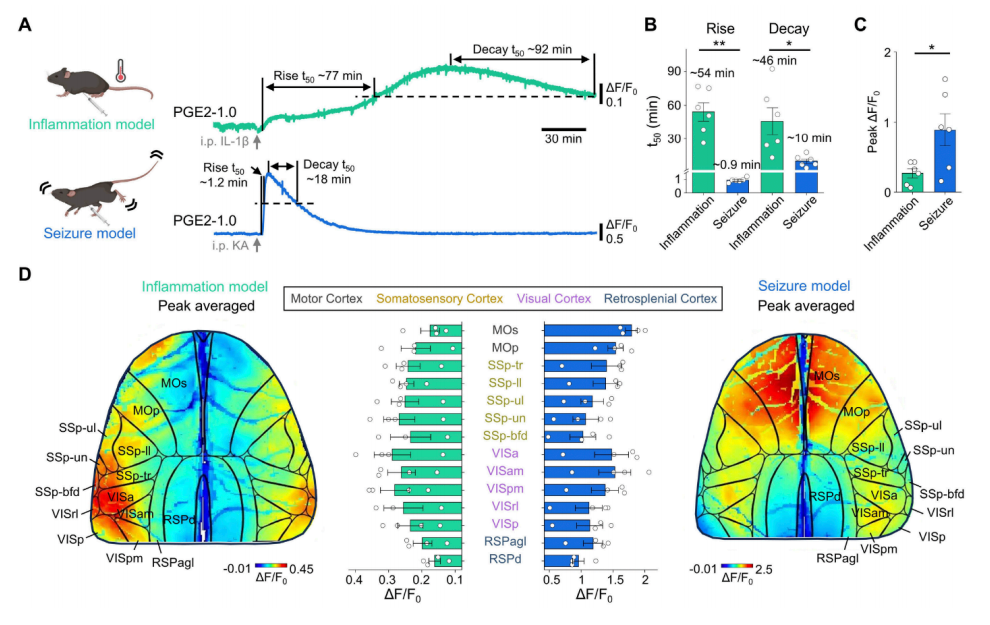 |
· Wang, L., Yang, Y., Deng, F., Yan, Y., & Li, Y.*. (2025).
A genetically encoded fluorescent sensor for monitoring spatiotemporal prostaglandin E2 dynamics in vivo.
bioRxiv.
[Full Text]
[PDF] |
|
 |
· Xie, S., Miao, X., Li, G., Zheng, Y., Li, M., Ji, E., Wang, J., Li, S., Cai, R., Geng, L., Feng, J., Wei, C.*, & Li, Y.*. (2024).
Red-shifted GRAB acetylcholine sensors for multiplex imaging in vivo.
bioRxiv.
[Full Text]
[PDF] |
|
 |
· Lopez, R., Noble, N., Ozcete, O., Cai, X., Handy, G., Andersen, J., Patriarchi, T., Li, Y.*, & Kaeser, P.* (2024).
Innervation density governs crosstalk of GPCR-based norepinephrine and dopamine sensors.
bioRxiv.
[Full Text]
[PDF] |
|
 |
· Wang T., Zhang X., Duan H., Xia D., Li T., Yan R., Zhan Y., Li, Y., Gao W., & Zhou, Q.* (2024).
Gating of Memory to Behavior by the Locus Coeruleus.
bioRxiv, 2024.01.09.574947.
[Full Text]
[PDF] |
|
 |
· Ge, C., Chen, Z., Sun, F., Hou, R., Fan, H., Li, Y., & Li, C.* (2024).
Timing-dependent modulation of working memory by VTA dopamine release in medial prefrontal cortex.
bioRxiv, 2024.09.11.611894.
[Full Text]
[PDF] |
|
 |
· Wu, Y., Gu, X., Kong, Y., Yang, S., Wang, H., Xu, M., Wang, Q., Yi, X., Lin, Z., Jiao, Z., Cheung, H., Zhao, X., Bian, X., Jiang, Q., Li, Y., Zhu, M., Wang, L., Li, Y., Huang, J., Li, Q., Li, W., & Xu, T.* (2024).
Neuropeptide Y co-opts neuronal ensembles for memory lability and stability.
bioRxiv, 2024.05.09.593455.
[Full Text]
[PDF] |
|
 |
· Garcia, S., Laffere, A., Toschi, C., Schilling, L., Podlaski, J., Fritsche, M., Zatka-Haas, P., Li, Y., Bogacz, R., Saxe, A., & Lak, A.* (2023).
Striatal dopamine reflects individual long-term learning trajectories.
bioRxiv, 2023.12.14.571653.
[Full Text]
[PDF] |
|
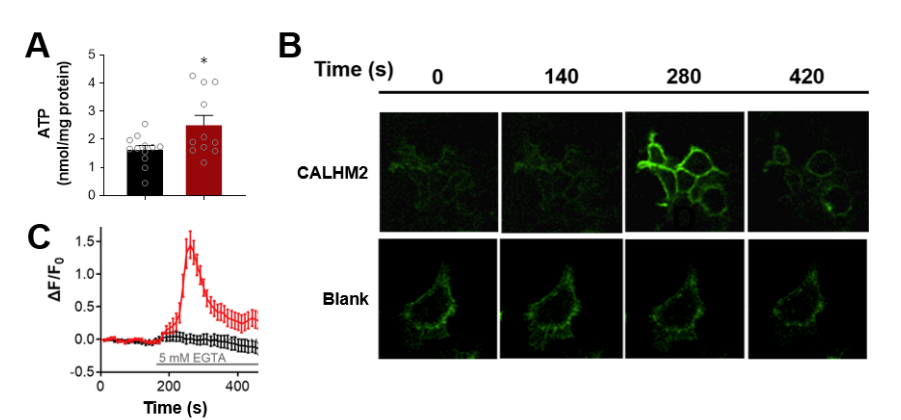 |
· Guo, Q., Hou, T., Xie, W., Zhang, J., Ma, X., Guo, Y., Wang, X., Wang, L., Lu, M., Wu, Z, Wang, H., Chen, Y., Li, Y., & Wang, S.*(2024).
Calcium Homeostasis Modulator 2 Constitutes an ATP-regulation Pore in Mitochondria.
bioRxiv, 2024.09.30.615983.
[Full Text]
[PDF] |
|
 |
· Yang, J., Basu, A., Liu, R., Staszko, S., Yu, A., Rondeau, J., Glaeser-Khan, S., Feng, J., Li, Y., Che, A., & Kaye, A.* (2023).
Frontal cortex norepinephrine, serotonin, and dopamine dynamics in an innate fear-reward behavioral model.
bioRxiv, 2023.11.27.568929.
[Full Text]
[PDF] |
|
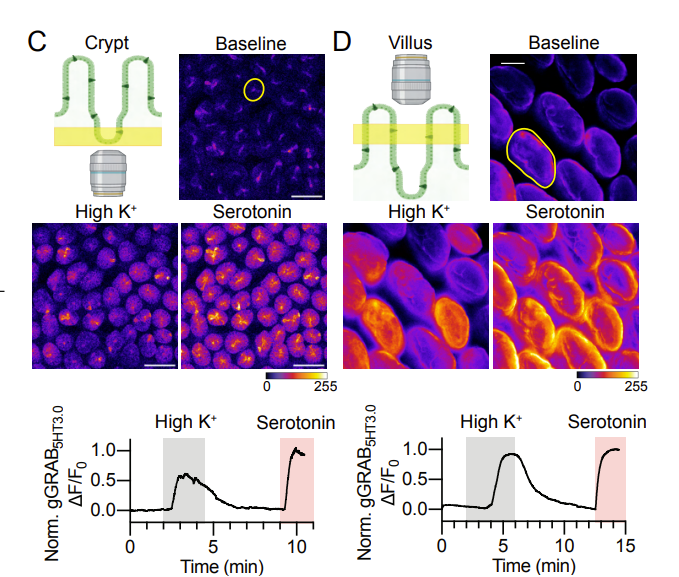 |
· Touhara, K., Rossen, N., Deng, F., Chu, T., Harrington, A., Caraballo, S., Brizuela, M., O'Donnell, T., Cil, O., Brierley, S., Li, Y., & Julius, D.* (2024).
Crypt and Villus Enterochromaffin Cells are Distinct Stress Sensors in the Gut.
bioRxiv, 2024.02.06.579180.
[Full Text]
[PDF] |
|
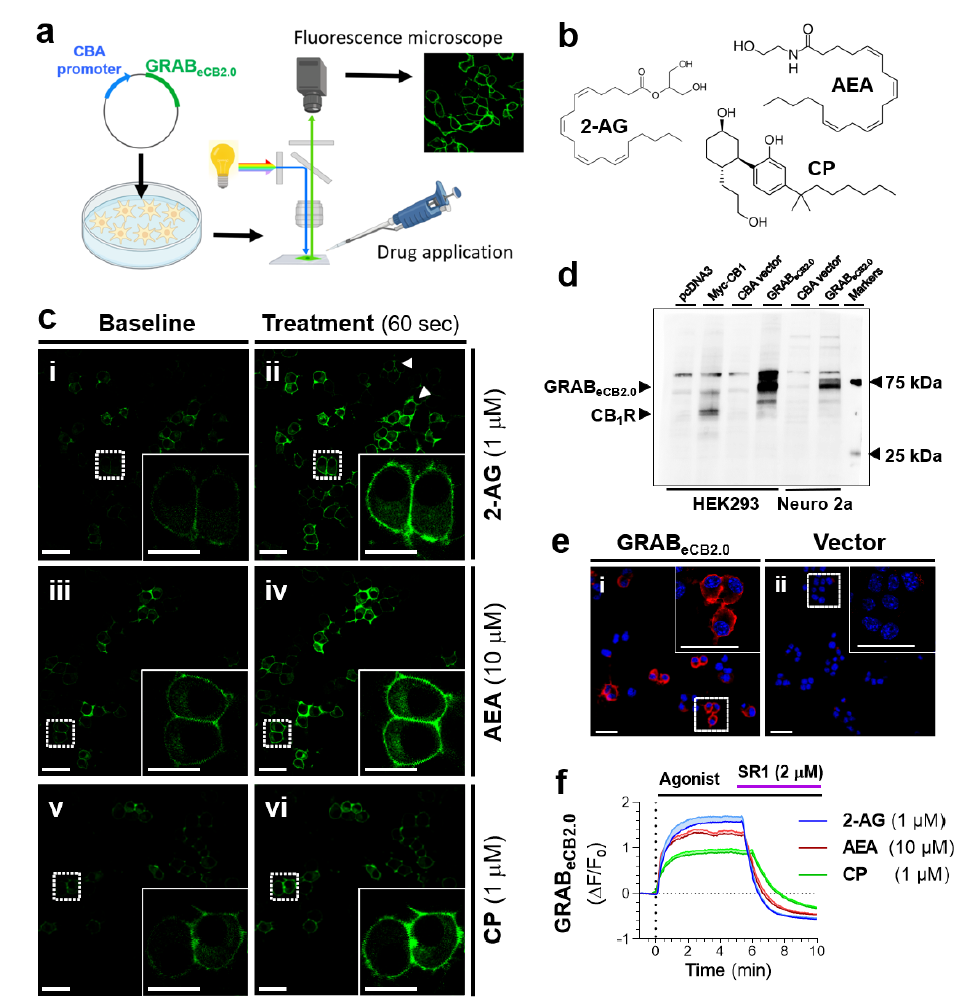 |
· Singh, S., Sarroza, D., English, A., Whittington, D., Dong, A., van der Stelt, M., Li, Y., Zweifel, L., Bruchas, M. R., Land, B. B., & Stella, N.* (2022).
ABHD6 selectively controls metabotropic-dependent increases in 2-AG production.
bioRxiv, 2024.2005.2017.594562.
[Full Text]
[PDF] |
|
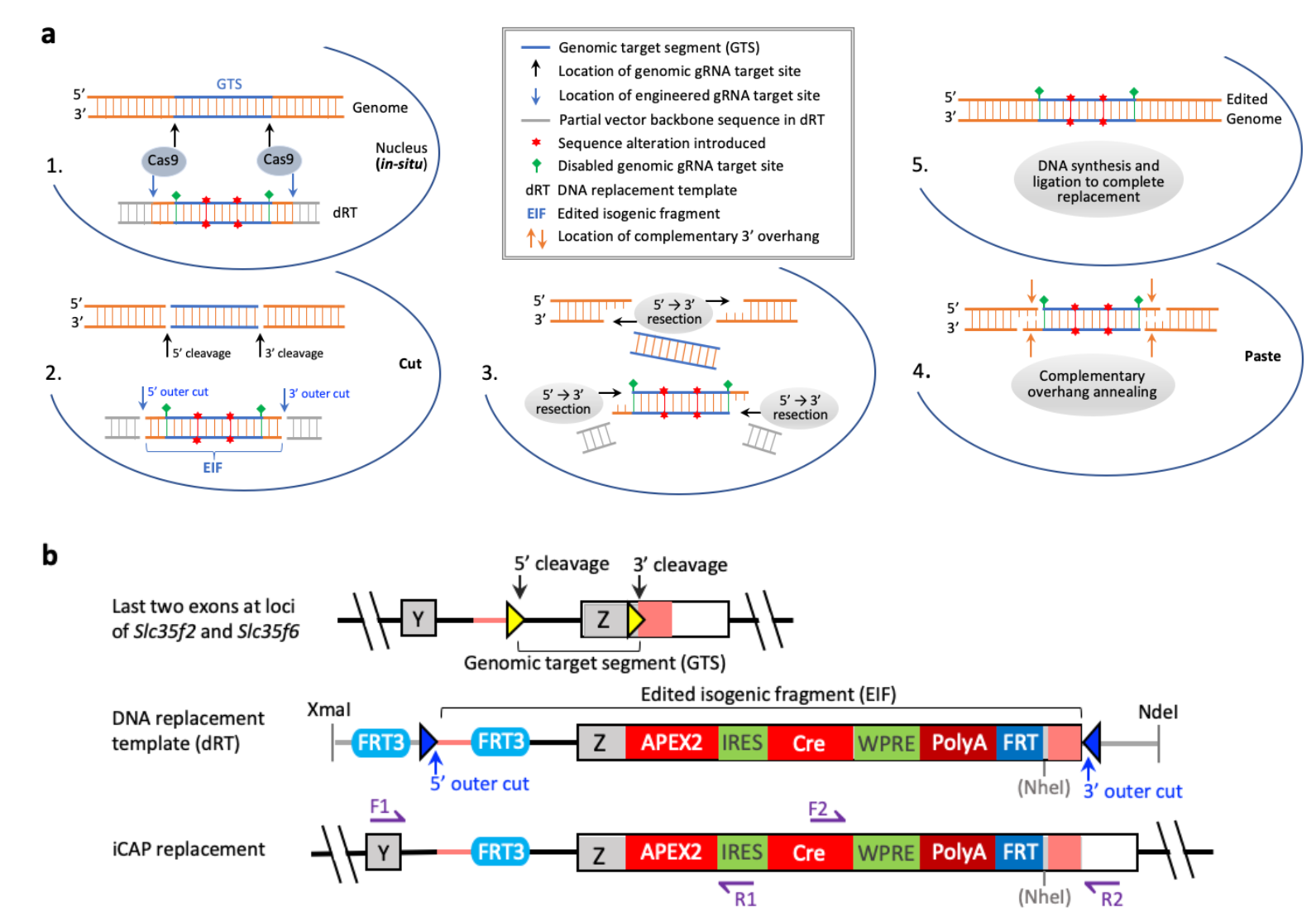 |
· Jiang, P.*, Kemper, K. M., Chang, K.-T., Qian, C., Li, Y., Guan, L., van Hasselt, P., Caradonna, S. J., & Strich, R. (2022).
An in situ cut-and-paste genome editing platform mediated by CRISPR/Cas9 or Cas12a.
bioRxiv, 2022.2003.2030.486486.
[Full Text]
[PDF] |
|
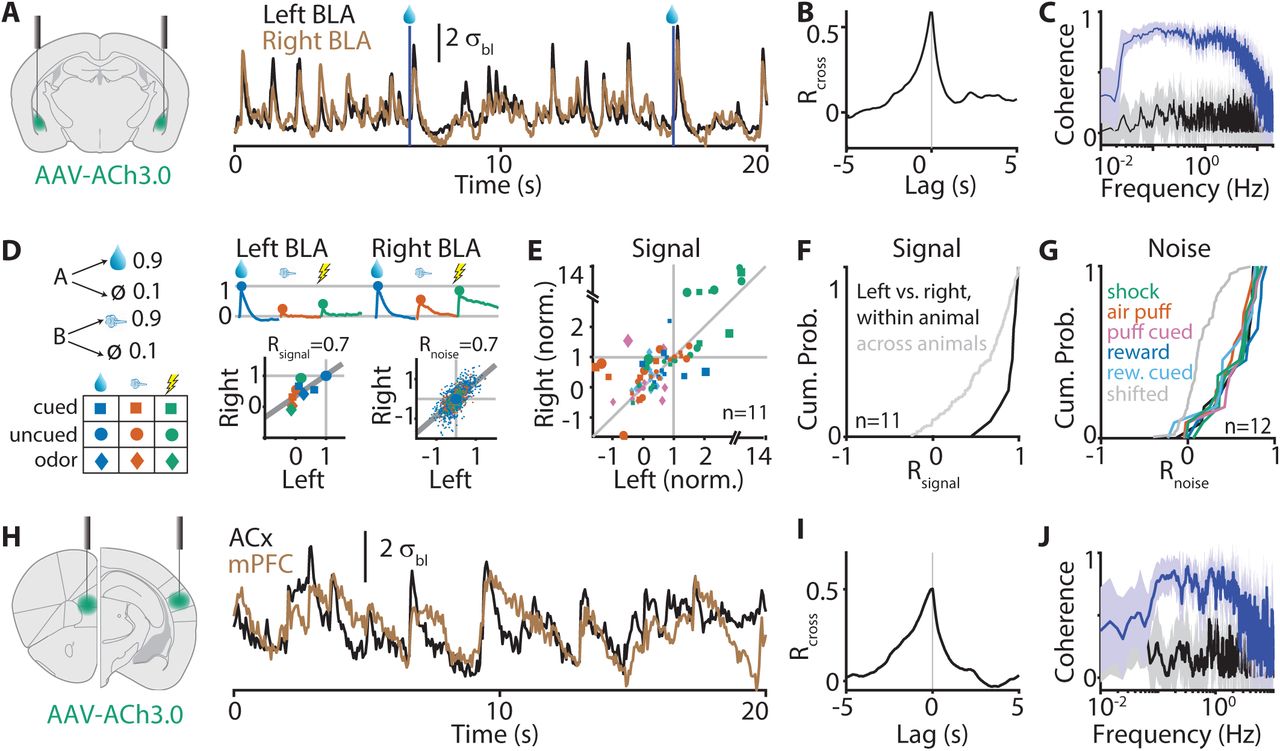 |
· Sturgill, J. F., Hegedus, P., Li, S. J., Chevy, Q, Siebels, A., Jing, M., Li, Y., Hangya, B.* & Kepecs, A.*(2020). Basal forebrain-derived acetylcholine encodes valence-free reinforcement prediction error. bioRxiv, 2020.02.17.953141.
[Full Text]
[PDF] |
合作发表论文
 |
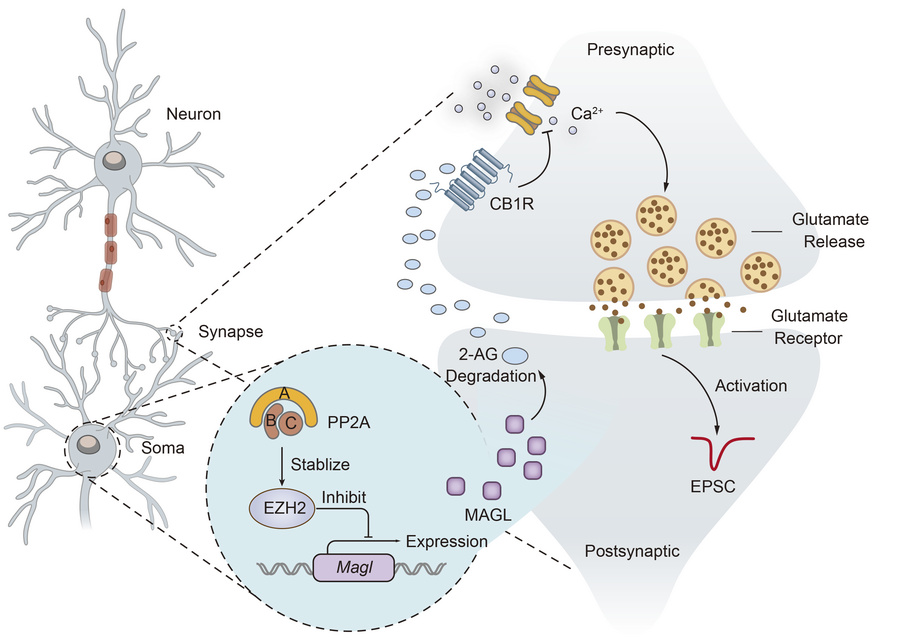
· Klose, M., Kim, J., Gregg, S., Schmidt, B., Xia, X., Li, Y., Levitan, E. (2026)
Ppp2r1a haploinsufficiency increases excitatory synaptic transmission and decreases spatial learning by impairing endocannabinoid signaling.
Current Biology.
[Full Text]
[PDF] |
|
 |
· Wang, Y., Duan, W., Li, H., Tang, Z., Cai, R, Cai, S., Deng, G., Chen, L., Luo, H., Chen, L., Li, Y., Wang, J., Xiong, B., & Jiang, M.*(2025)
Ppp2r1a haploinsufficiency increases excitatory synaptic transmission and decreases spatial learning by impairing endocannabinoid signaling.
The Journal of Clinical Investigation.
[Full Text]
[PDF] |
|
 |
· Fan, J., Wang, Y., Li, L., He, J., Zhao, Z., Deng, F., Li, G., Li, X., Zhou, Y., Zhao, J., Huang, N., Hu, Y., Li, Y., Wu, J.*, Fang, L.* & Dai, Q.* (2025)
Prominent involvement of acetylcholine in shaping stable olfactory representation across the Drosophila brain.
Nature Communications.
[Full Text]
[PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2024.04.03.587915v1 |
|
 |
· Liu, Y., Nong, Y., Feng, J., Li, G., Sajda, P., Li, Y. & Wang, Q.* (2025)
Phase synchrony between prefrontal noradrenergic and cholinergic signals indexes inhibitory control.
Nature Communications.
[Full Text]
[PDF] See also BioRxiv https://doi.org/10.1101/2024.05.17.594562 |
|
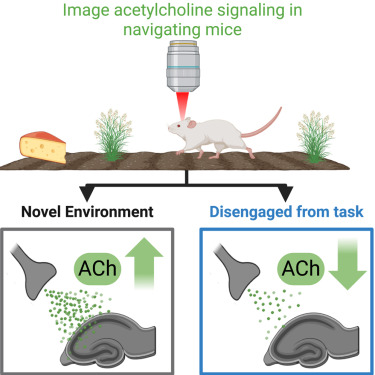 |
· Xuan, F., Li, G., Li, Y., & Dombeck, D.* (2025)
Modulation of speed-dependent acetylcholine release in the hippocampus by spatial task engagement.
Cell Reports. Volume 44, Issue 10.
[Full Text]
[PDF] |
|
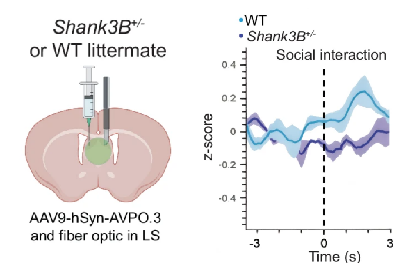 |
· Bortolozzo-Gleich, M., Bouisset, G., Geng, L., Pino, A., Nomura, Y., Han, S., Li, Y., & Leroy, F.* (2025)
Impaired vasopressin neuromodulation of the lateral septum leads to social behavior deficits in Shank3B+/- male mice.
Nature Communications.
[Full Text]
[PDF] |
|
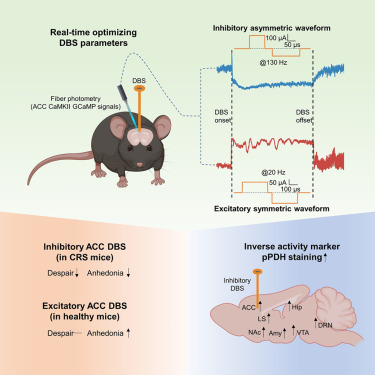 |
· Yuan, Z.*, Yang, H., Wang, P., Hou, X., Xu, K., Zhou, Y., Dai, R., Gao, Y., Gao, X., Guo, Q., Li, Y., Zhang, J., Mao, Z.*, & Luo, M.* (2025)
Optimized deep brain stimulation for anterior cingulate cortex inhibition produces antidepressant-like effects in mice.
Neuron.
[Full Text]
[PDF] |
|
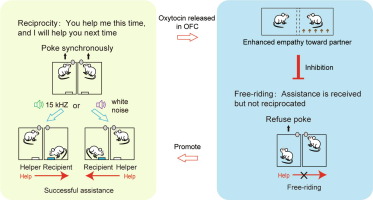 |
· Wang, M., Shi, Q., Shao, Y., Jiang, M., Fu, A., Huang, Y., Wang, Q., Wei, L., Zhang, Z., Xu, J., Yang, T., Li, Y.*, & Wang, Z.* (2025)
Oxytocin-mediated empathy internallyfacilitates cooperative behaviors in rats.
Science Bulletin.
[Full Text]
[PDF] |
|
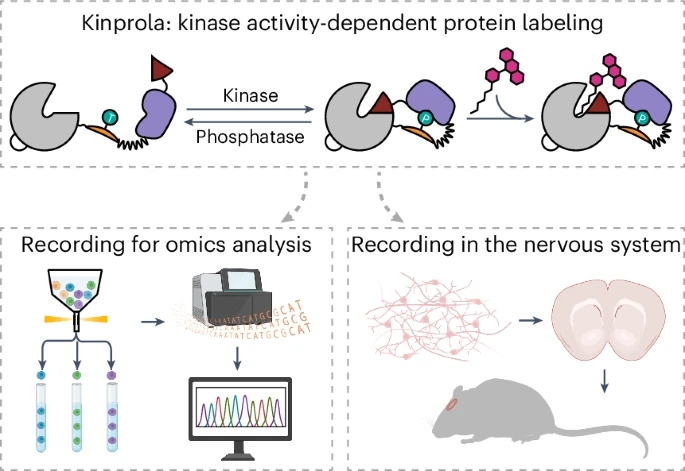 |
· Sun, D.*, Ng, S., Zheng, Y., Xie, S., Schwan, N., Breuer, P., Hoffmann, D., Michel, J., Azorin, D., Boonekamp, K., Winkler, F., Wick, W., Boutros, M., Li, Y. & Johnsson, K.* (2025)
Molecular recording of cellular protein kinase activity with chemical labeling.
Nature Chemical Biology.
[Full Text]
[PDF] |
|
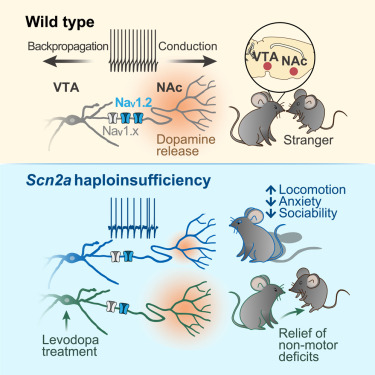 |
· Li, L.#, Huang, Q.#, Hu, J., Jin, M., Zhuo, Y., Ke, W., He, Q., Xiao, Y., Zhang, X., Wang, W., Cheng, T., Tai, Y., Guo, F., Yu, J., Li, Y., He, J., Li, B., & Shu, Y.* (2025)
Selective loss of Scn2a in ventral tegmental area dopaminergic neurons leads to dopamine system hypofunction and autistic-like behaviors.
Neuron.
[Full Text]
[PDF] |
|
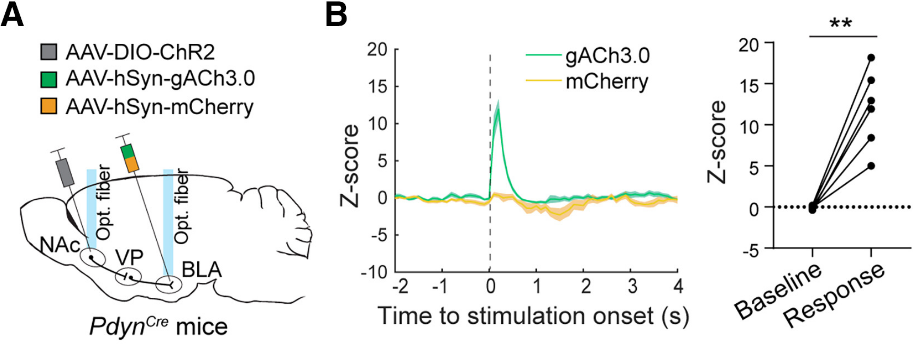 |
· Sun, Q.#*, Liu, M.#, Guan, W., Xiao, X., Dong, C., Bruchas, M., Zweife, L., Li, Y., Tian, L., & Li, B.* (2025)
Dynorphin modulates reward-seeking actions through a pallido-amygdala cholinergic circuit.
Neuron.
[Full Text]
[PDF] |
|
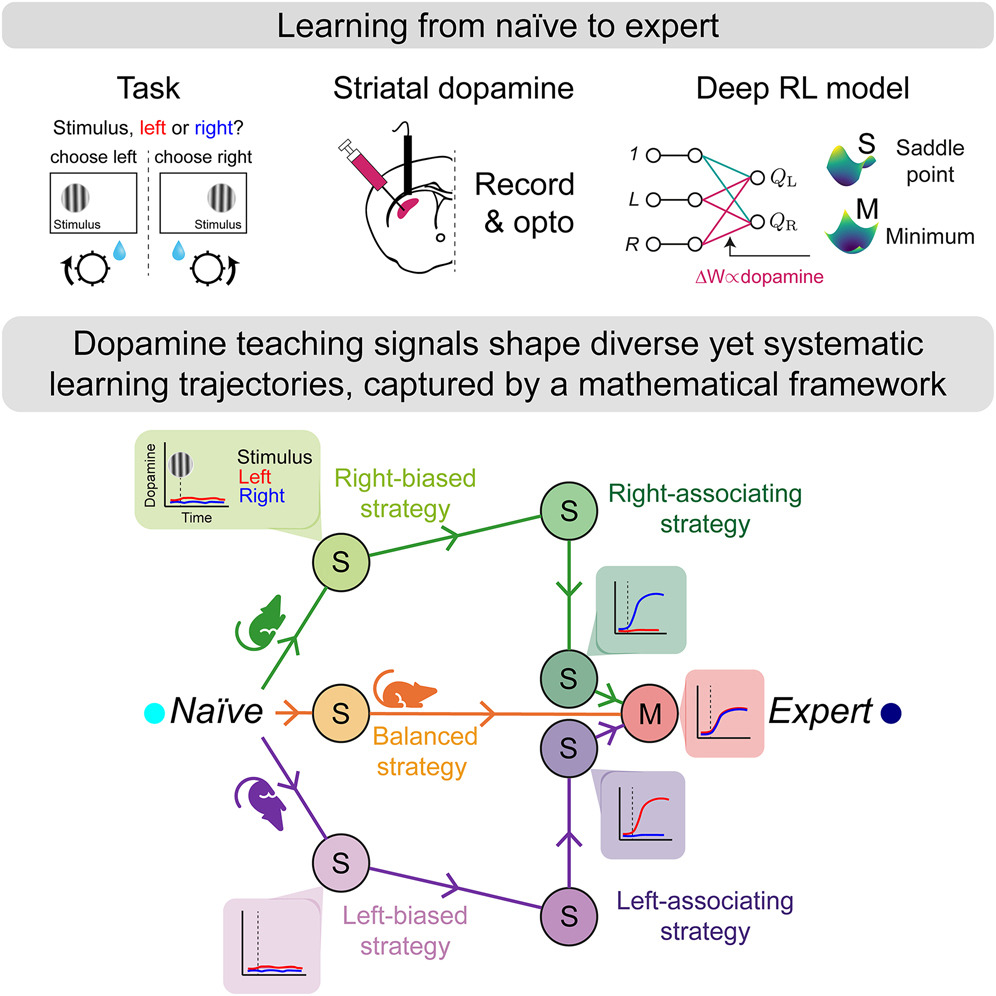 |
· Liebana, S.*, Laffere, A., Toschi, C., Schilling, L., Moretti, J., Podlaski, J., Fritsche, M., Zatka-Haas, P., Li, Y., Bogacz, R., Saxe, A. & Lak, A.* (2025)
Dopamine encodes deep network teaching signals for individual learning trajectories.
Cell.
[Full Text]
[PDF] |
|
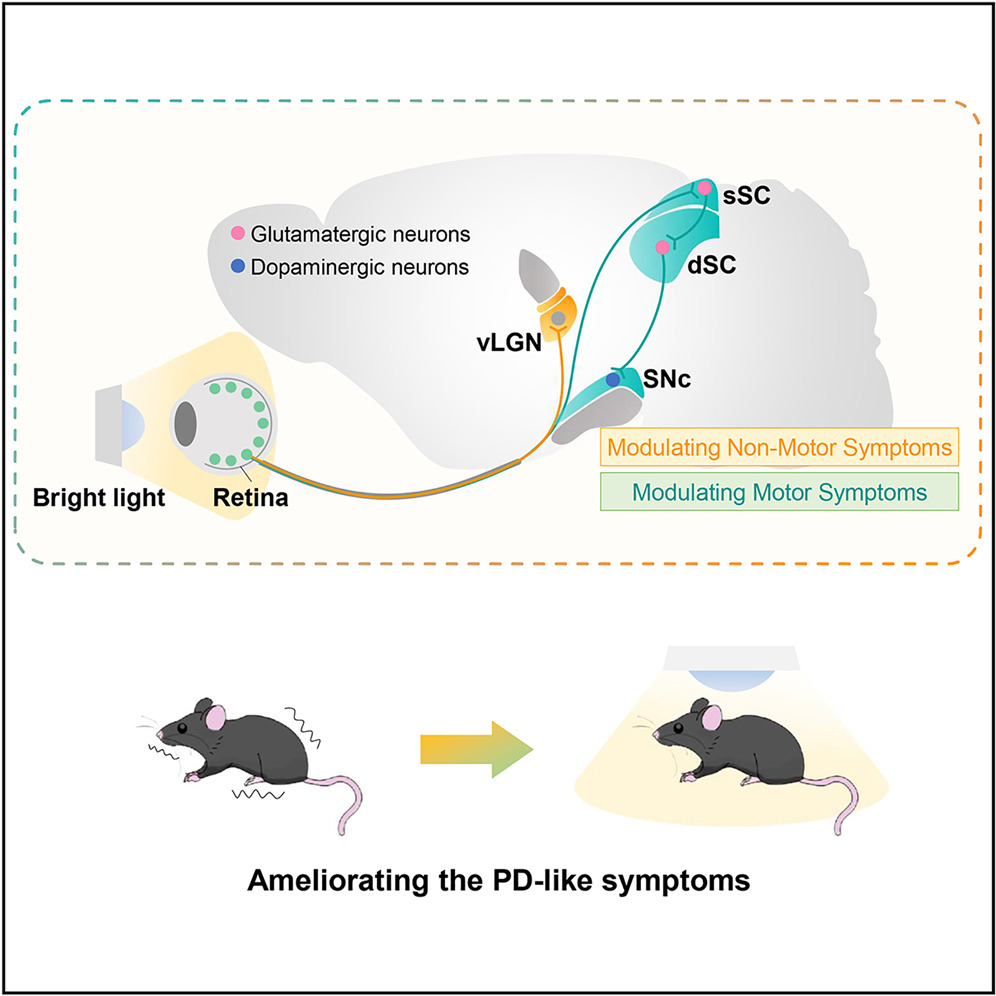 |
· Huang, X.#*, Wang, S.#, Chen, Z.#, Qu, W., Song, L., Hu, Z., Xi, Y., Yang, Y., Hong, W., Lin, S, So, K., Li, Y., Huang, L.*, Tao, Q.*, & Ren, C.* (2025)
Bright-light treatment ameliorates motor and non-motor deficits through distinct visual circuits in a mouse model of Parkinson's disease.
Cell Reports. Volume 44, Issue 6, 2025.
[Full Text]
[PDF] |
|
 |
· Wei, Q., Bai, Z., Wang, L., Wang, J., Wang, Y., Hu, Y., Ding, S., Ma, Z., Li, C., Li, Y., Zhuo, Y., Li, W., Deng, F., Liu, B., Zhou, P., Li, Y.*, Wu, Z.* & Wang, J.* (2025)
A high-performance fluorescent sensor spatiotemporally reveals cell-type specific regulation of intracellular adenosine in vivo.
Nature Communications. 16, 4245(2025)
[Full Text]
[PDF] |
|
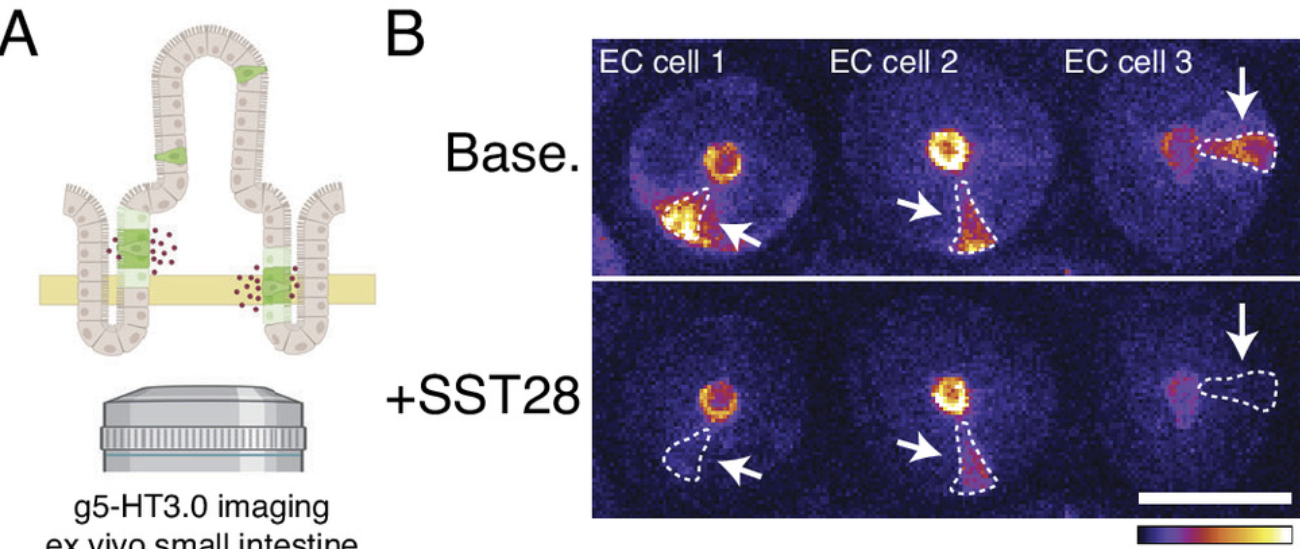 |
· Rossen, N., Touhara, K., Castro, J., Harrington, A., Caraballo, S., Deng, F., Li, Y., Brierley, S. & Julius, D.* (2025)
Population imaging of enterochromaffin cell activity reveals regulation by somatostatin.
PNAS. 122 (19) e2501525122.
[Full Text]
[PDF] |
|
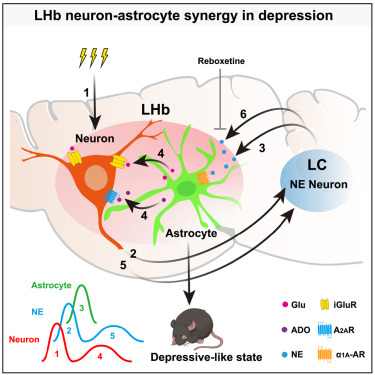 |
· Xin, Q.#, Wang, J.#, Zheng, J., Tan, Y., Jia, X., Ni, Z., Xu, Z., Jiesi Feng, Zhaofa Wu, Li, Y., Li, X., Ma, H., & Hu, H.* (2025)
Neuron-astrocyte coupling in lateral habenula mediates depressive-like behaviors.
Cell.
[Full Text]
[PDF] |
|
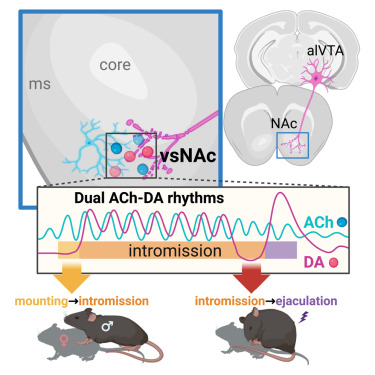 |
· Miyasaka, A., Kanda, T., Nonaka, N., Terakoshi, Y., Cherasse, Y., Ishikawa, Y., Li, Y.,
Takizawa, H., Hirano, A., Seita J., Yanagisawa M., Sakurai, T.*, Sakurai, K.*, & Liu, Q.* (2025)
Sequential transitions of male sexual behaviors driven by dual acetylcholine-dopamine dynamics.
Neuron.
[Full Text]
[PDF] |
|
 |
· Tsutsui-Kimura, I., Tian, Z., Amo, R., Zhuo, Y., Li, Y., Campbell, M., Uchida, N., & Watabe-Uchida, M.* (2025)
Dopamine in the tail of the striatum facilitates avoidance in threat-reward conflicts.
Nature Neuroscience.
[Full Text]
[PDF] |
|
 |
· Costa, K.*, Zhang, Z.*, Deutsch, D., Zhuo, Y., Li, G., Li, Y., & Schoenbaum, G.* (2025)
Dopamine and acetylcholine correlations in the nucleus accumbens depend on behavioral task states.
Current Biology.
[Full Text]
[PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2024.05.03.592439v1 |
|
 |
· Straub, V., Barti, B., Tandar, S., Stevens, A., van der Wel, T., Zhu, N., Rüegger, J., van der Horst, C., Heitman, L. Li, Y., Stella, N., van Hasselt, J. G., Coen Katona, I., & van der Stelt, M.* (2025)
The endocannabinoid 2-arachidonoylglycerol is released and transported on demand via extracellular microvesicles.
PNAS. 122 (8) e2421717122.
[Full Text]
[PDF] See also BioRxiv https://doi.org/10.1101/2024.09.23.614520 |
|
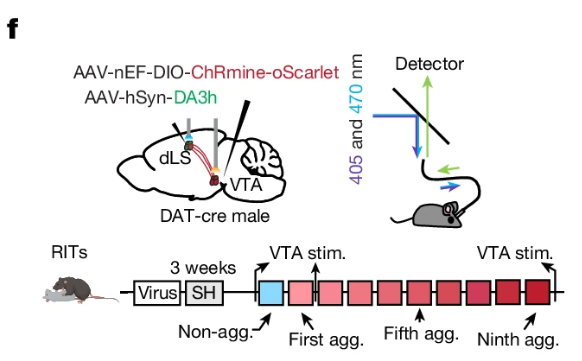 |
· Dai, B.* , Zheng, B., Dai, X., Cui, X., Yin, L., Cai, J., Zhuo, Y., Tritsch, N., Zweifel, L., Li, Y. & Lin, D.* (2024)
Experience-dependent dopamine modulation of male aggression.
Nature.
[Full Text]
[PDF] |
|
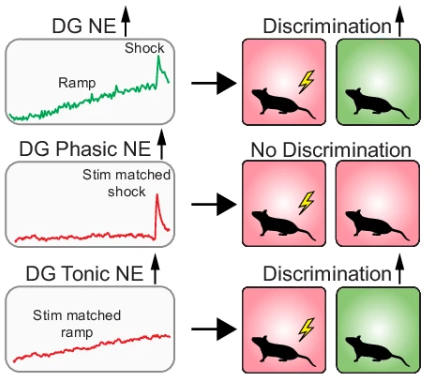 |
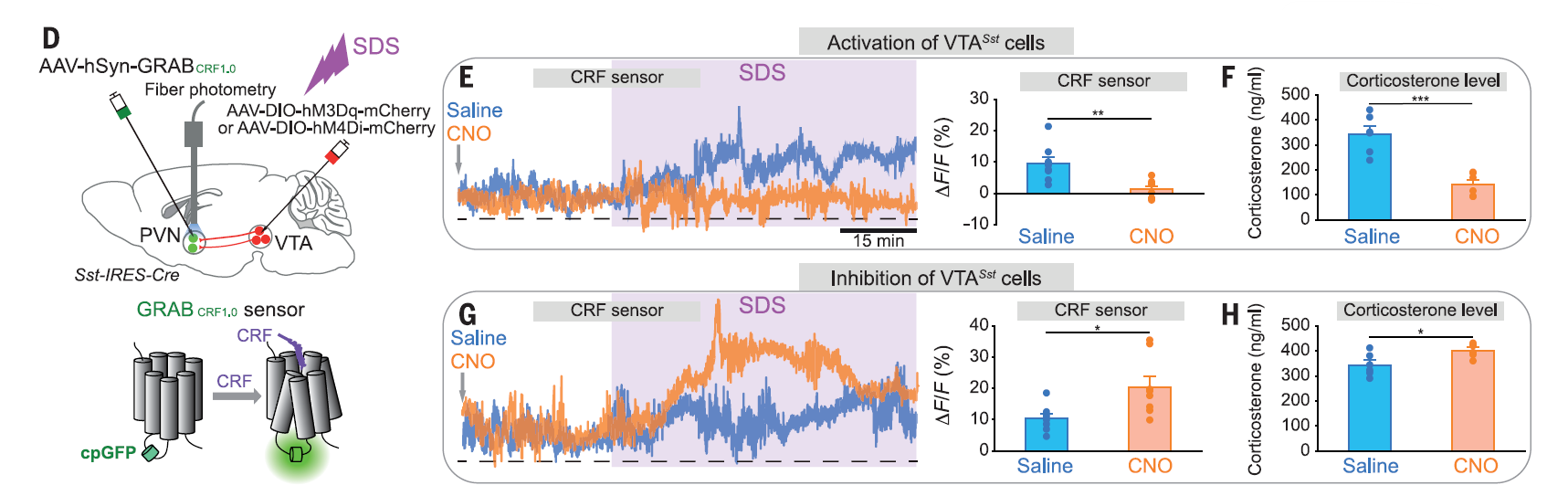
· Zhang, E., Saglimbeni, G., Feng, J. , Li, Y. & Bruchas, M.* (2024)
Dentate gyrus norepinephrine ramping facilitates aversive contextual processing.
Nature Communications.
[Full Text]
[PDF] |
|
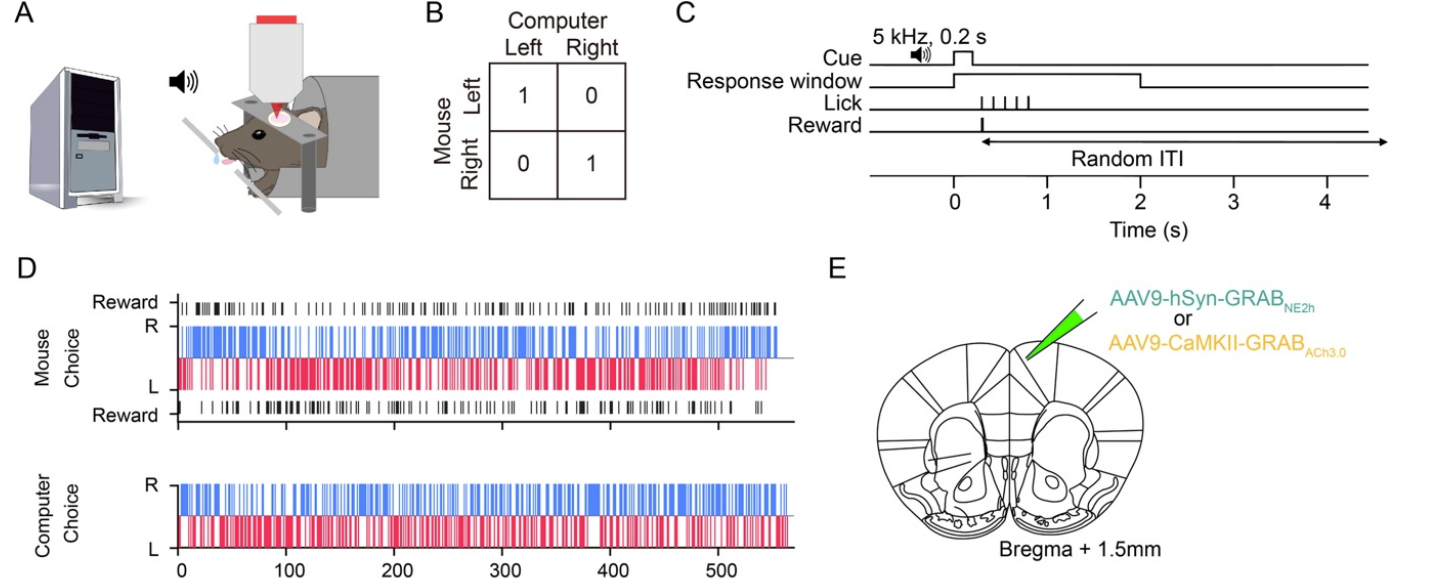 |
· Wang, H., Ortega, H., Kelly, E., Indajang, E., Feng, J. , Li, Y., & Kwan, A.* (2024).
Frontal noradrenergic and cholinergic transients exhibit distinct spatiotemporal dynamics during competitive decision-making.
Science Advances, Vol 11, Issue 13.
[Full Text]
[PDF] |
|
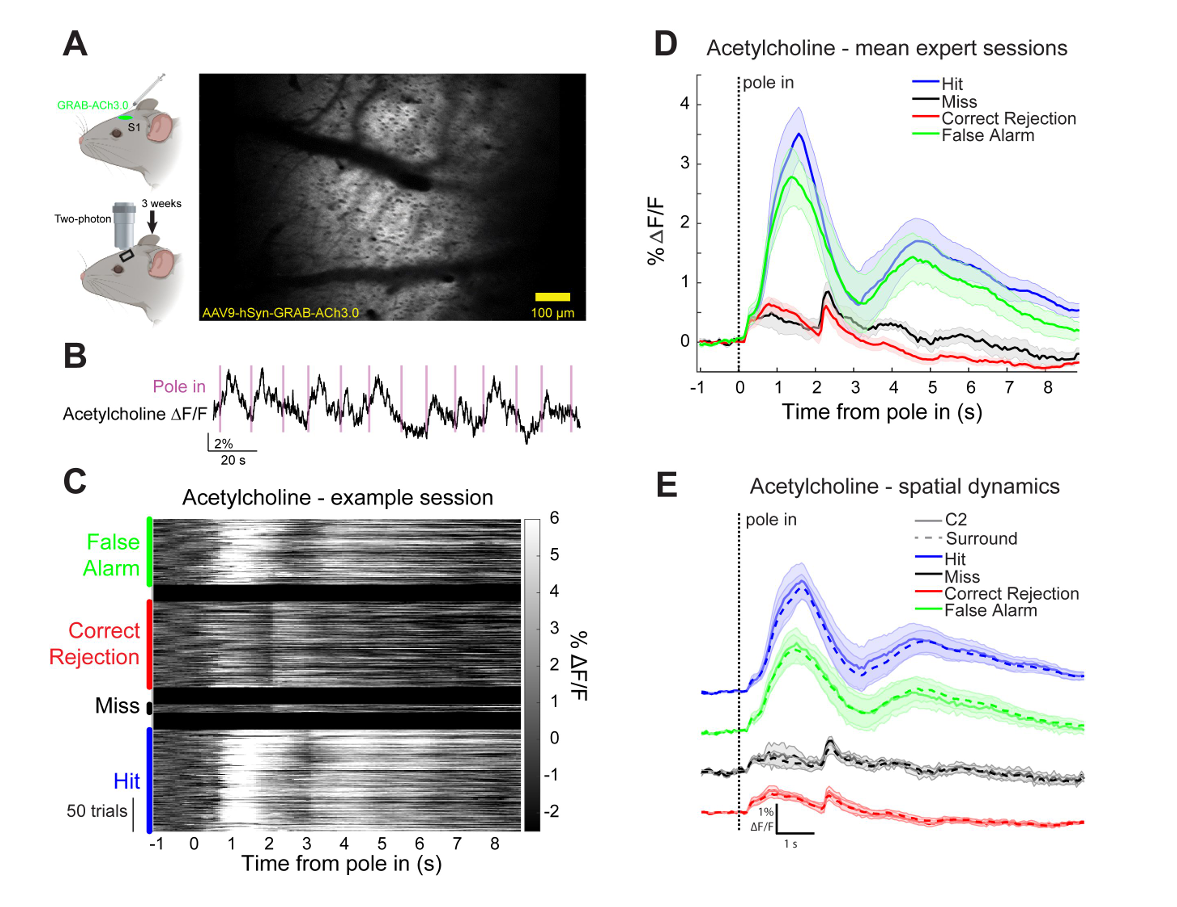 |
· Zou, J., Willem, J., Mridha, Z., Trinh, S., Erskine, A., Jing, M., Yao, J., Walker, S., Li, Y., McGinley, M., Hires, S.* (2024)
Goal-directed motor actions drive acetylcholine dynamics in sensory cortex
eLife , 13:RP96931
[Full Text]
[PDF] |
|
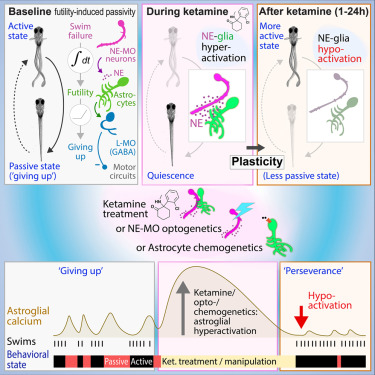 |
· Duque, M.*, Chen, A., Hsu, E., Narayan, S., Rymbek, A., Begum, S., Saher, G., Cohen, A., Olson, D., Li, Y., Prober, D., Bergles, D., Fishman, M., Engert, F., & Ahrens, M.* (2024)
Ketamine induces plasticity in a norepinephrine-astroglial circuit to promote behavioral perseverance.
Neuron.
[Full Text]
[PDF] |
|
 |
· Ji, E., Zhang, Y., Li, Z. , Wei, L., Wu, Z., Li, Y., Yu, X. & Song, T. (2024)
The Chemokine CCL2 Promotes Excitatory Synaptic Transmission in Hippocampal Neurons via GluA1 Subunit Trafficking.
Neuroscience Bulletin.
[Full Text]
[PDF] |
|
 |
· Kalogriopoulos, N., Tei, R., Yan, Y., Klein, P., Ravalin, M., Cai, B., Soltesz, I., Li, Y., & Ting, A.* (2024)
Synthetic GPCRs for programmable sensing and control of cell behaviour.
Nature.
[Full Text]
[PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2024.04.15.589622v1 |
|
 |
· Li, H., Zhao, Y., Dai, R. , Geng, P., Weng, D., Wu, W., Yu, F., Lin, R., Wu, Z. , Li, Y., & Luo, M.* (2024)
Astrocytes release ATP/ADP and glutamate in flashes via vesicular exocytosis.
Molecular Psychiatry.
[Full Text]
[PDF] |
|
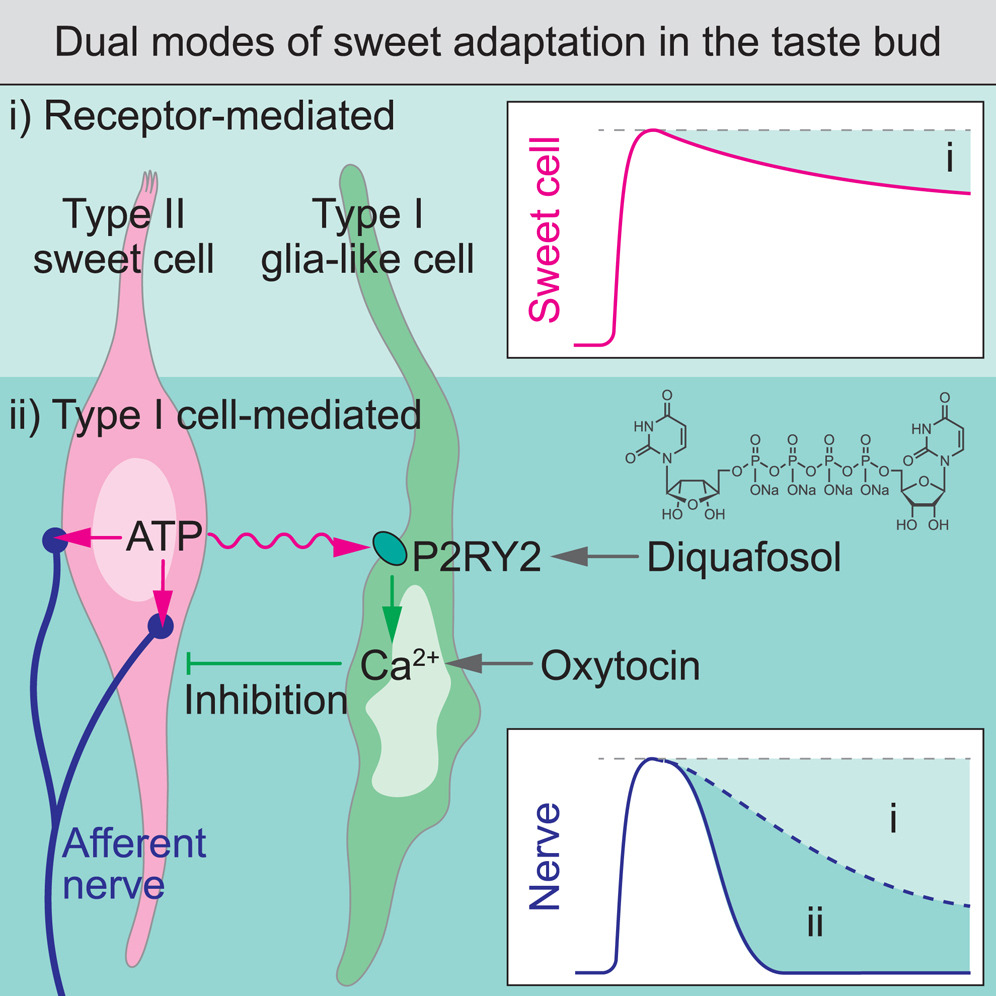 |
· Park, G., Lee, G., Yoon, J., Han, J., Choi, P., Kim, M., Lee, S., Park, C., Wu, Z. , Li, Y., & Choi, M.* (2024)
Glia-like taste cells mediate an intercellular mode of peripheral sweet adaptation.
Cell.
[Full Text]
[PDF] |
|
 |
· Cai, X., Liu, C., Tsutsui-Kimura, I., Lee, J., Guo, C., Banerjee, A., Lee, J., Amo, R., Xie, Y., Patriarchi, T., Li, Y., Watabe-Uchida, M., Uchida, N., & Kaeser, P.* (2024)
Dopamine dynamics are dispensable for movement but promote reward responses.
Nature.
[Full Text]
[PDF] |
|
 |
· Neyhart, E., Zhou, N., Munn, B., Law, R., Smith, C., Mridha, Z., Blanco, F., Li,G. , Li, Y., Hu, M., McGinley, M., Shine, J., & Reimer, J.*(2024)
Cortical acetylcholine dynamics are predicted by cholinergic axon activity and behavior state.
Cell Reports, Vol 43, Issue 10.
[Full Text]
[PDF] |
|
 |
· Berki, P., Cserép, C., Környei, Z., Pósfai, B., Szabadits, E., Domonkos, A., Kellermayer, A., Nyerges, M., Wei, X., Mody, I., Kunihiko, A., Beck, H., He K., Wang Y., Lénárt, N., Wu, Z., Jing, M., Li, Y., Gulyás, A., & Dénes, A.*(2024)
Microglia contribute to neuronal synchrony despite endogenous ATP-related phenotypic transformation in acute mouse brain slices.
Nature Communications, Vol. 15, Issue 1.
[Full Text]
[PDF] |
|
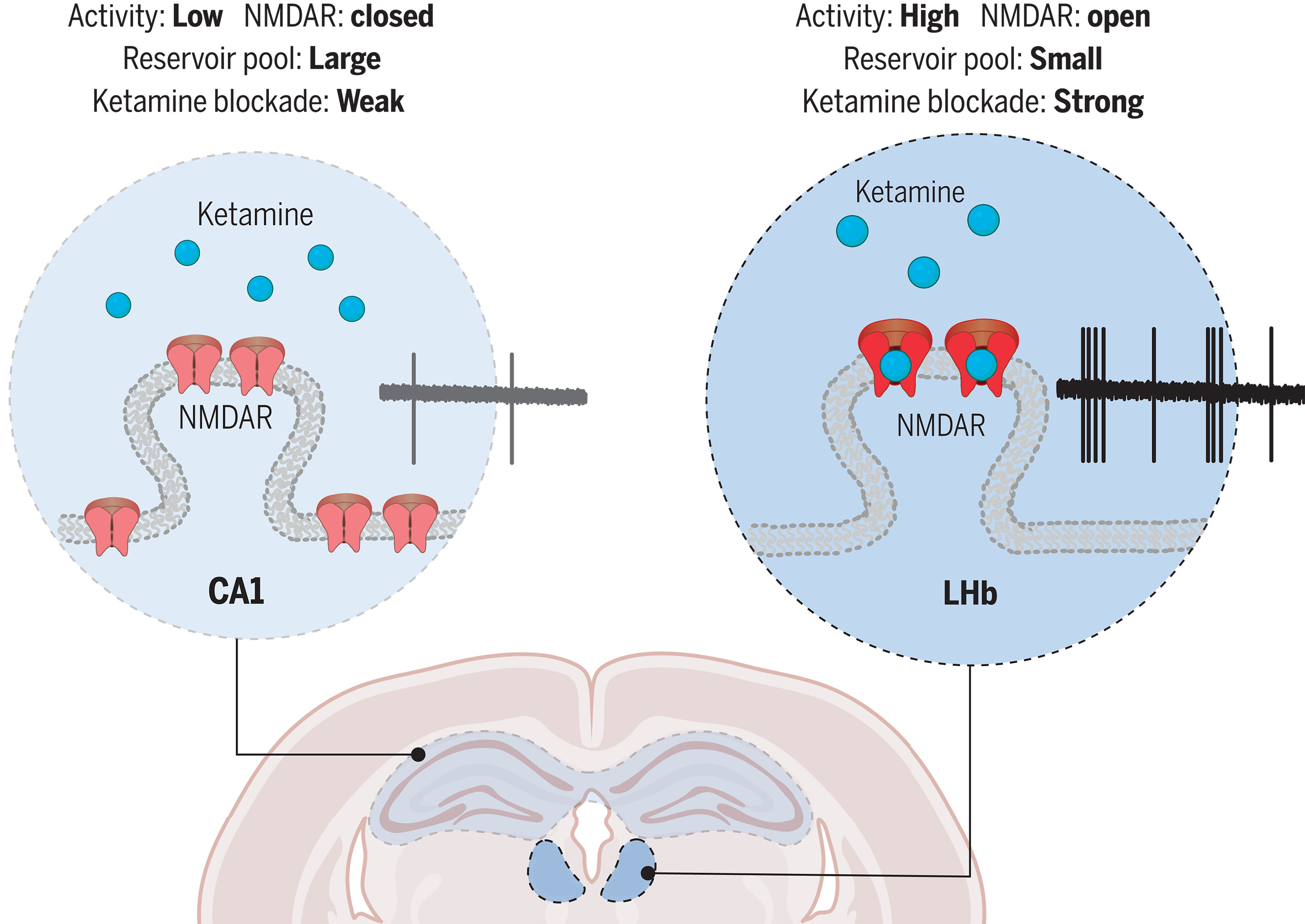 |
· Chen, M., Ma, S., Liu, H., Dong, Y., Tang, J., Ni, Z., Tan, Y., Duan, C., Li, H., Huang, H., Li, Y., Cao, X., J. Lingle, C., Yang Y., & Hu, H.*(2024)
Brain region–specific action of ketamine as a rapid antidepressant.
Science, Vol 385, Issue 6709.
[Full Text]
[PDF] |
|
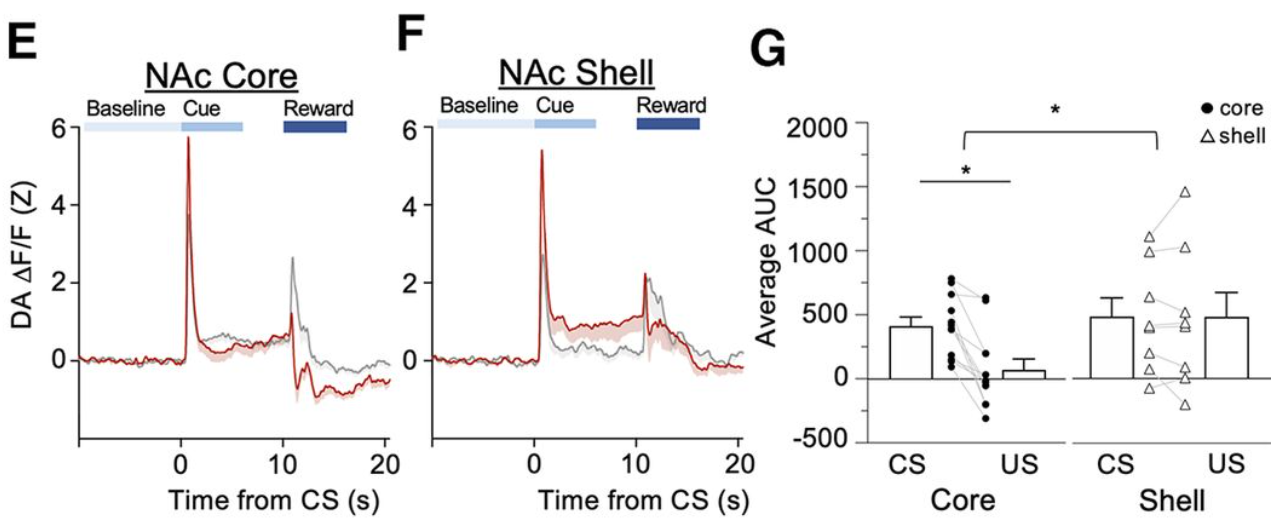 |
· Bacharach, S., Martin, D., Stapf, C., Sun, F., Li, Y., Cheer, J., & Calu, D.* (2023)
Decreased ventral tegmental area CB1R signaling reduces sign tracking and shifts cue-outcome dynamics in rat nucleus accumbens.
Journal of Neuroscience, 43 (25) 4684-4696
[Full Text]
[PDF] |
|
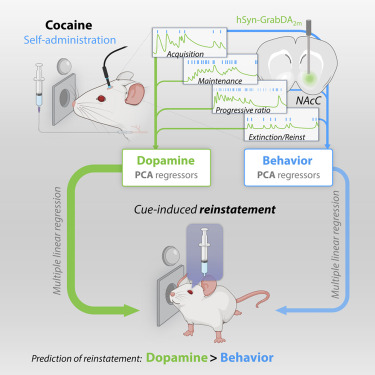 |
· Lujan, M., Oliver, B., Young-Morrison, R., Engi, S., Zhang, L., Wenzel, J., Li, Y., Zlebnik, N.* & Cheer, J.*(2023)
A multivariate regressor of patterned dopamine release predicts relapse to cocaine.
Cell Reports, Volume 42, Issue 6112553.
[Full Text]
[PDF] |
|
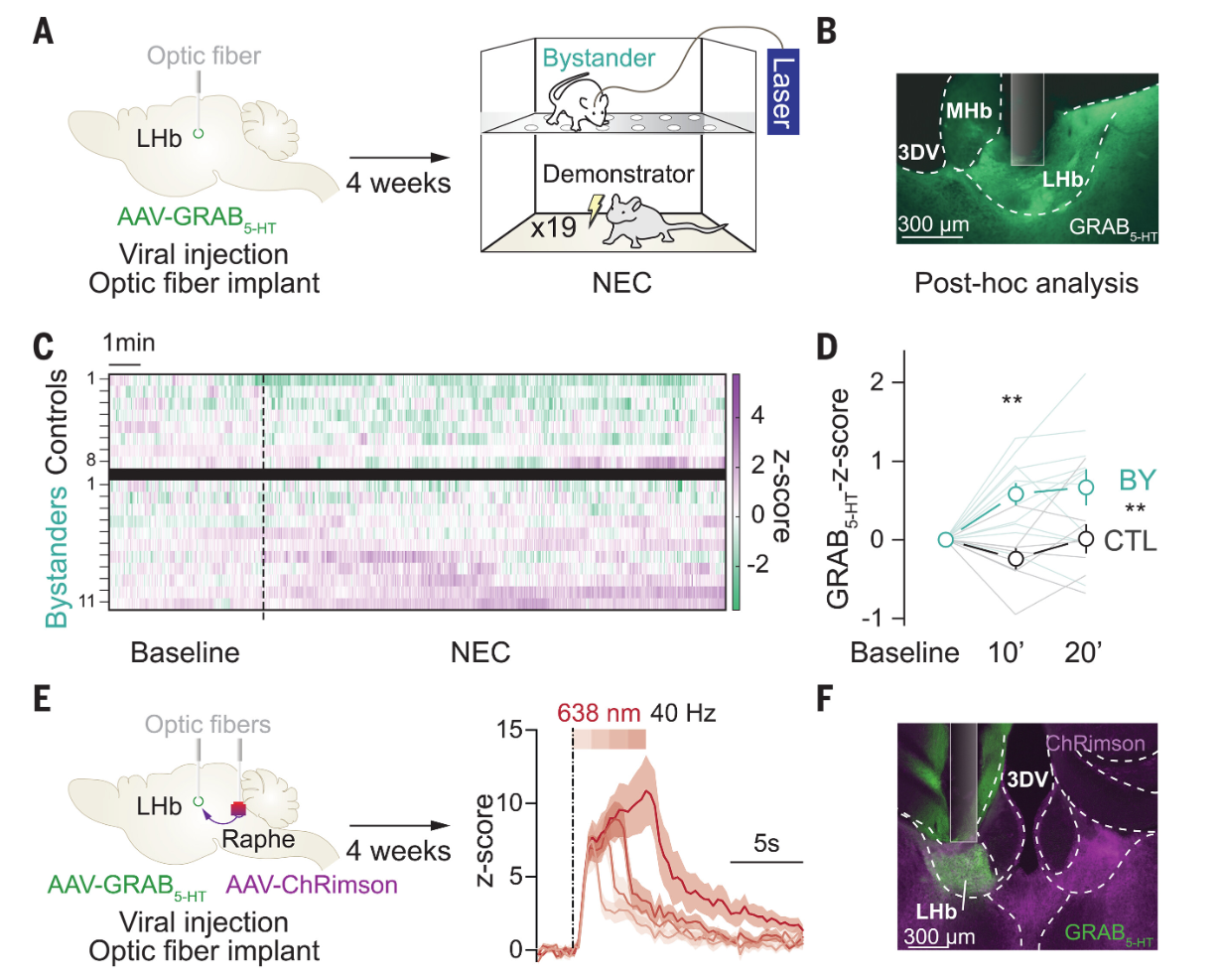 |
· Mondoloni, S., Molina, P., Lecca, S., Wu C., Michel, L., Osypenko, D., Cachin, F., Flanigan, M., Congiu, M., L. Lalive, A., Kash, T., Deng, F., Li, Y., & Mameli, M.*(2024)
Serotonin release in the habenula during emotional contagion promotes resilience.
Science, 1081-1086 .
[Full Text]
[PDF] |
|
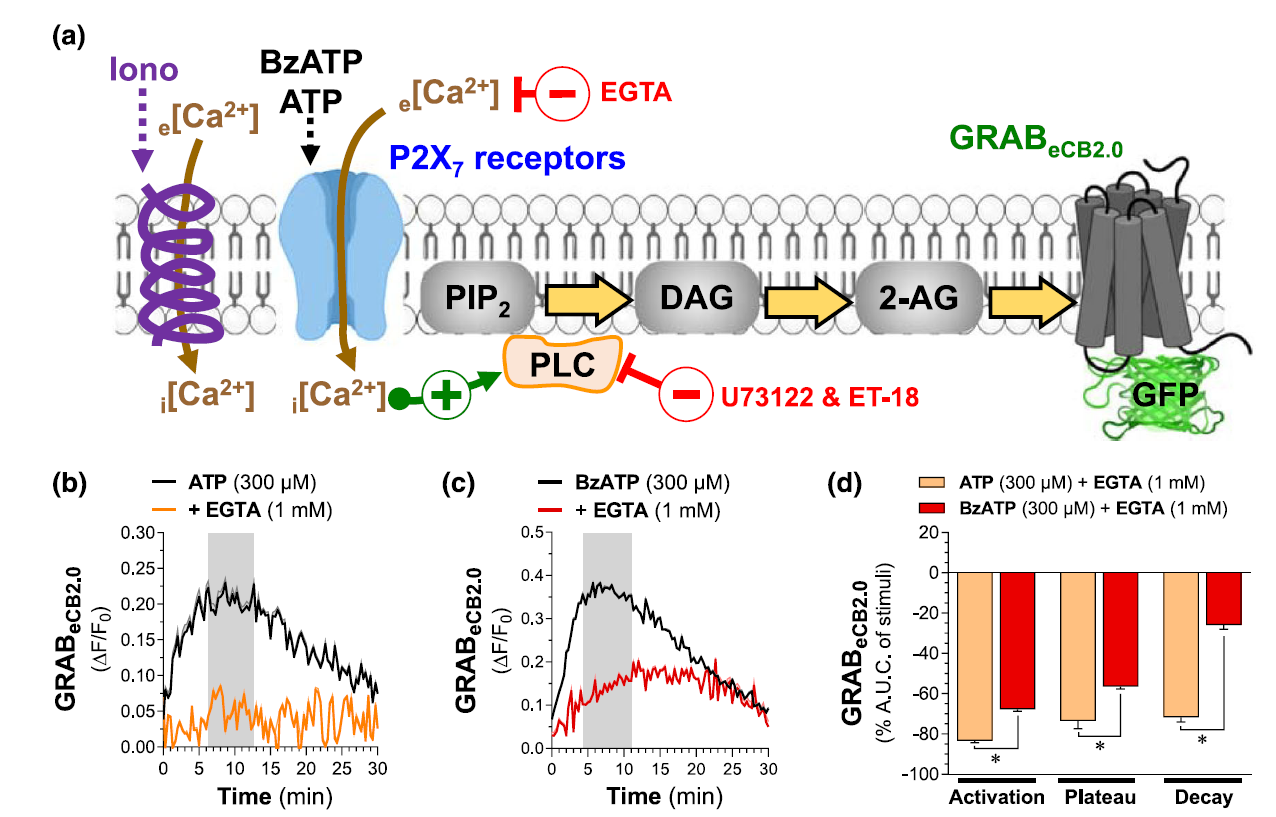 |
· Singh, S., Sarroza, D., English, A., Whittington, D., Dong, A., Malamas, M., Makriyannis, A., van der Stelt, M., Li, Y., Zweifel, L., Bruchas, M. R., Land, B. B., & Stella, N.* (2024)
P2X7 receptor-dependent increase in endocannabinoid 2-arachidonoyl glycerol production by neuronal cells in culture: Dynamics and mechanism.
British Journal of Pharmacology, 1–19.
[Full Text]
[PDF] |
|
 |
· Basu, A., Yang, J.-H., Yu, A., Glaeser-Khan, S., Rondeau, J. A., Feng, J., Krystal, J. H., Li, Y., & Kaye, A. P.* (2024).
Frontal Norepinephrine Represents a Threat Prediction Error Under Uncertainty.
Biological Psychiatry.
[Full Text]
[PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2022.10.13.511463 |
|
 |
· Glaeser-Khan, S., Savalia, N., Cressy, J., Feng, J., Li, Y., Kwan, A., & Kaye, A.* (2024).
Spatiotemporal organization of prefrontal norepinephrine influences neuronal activity.
eNeuro. 11(5).
[Full Text]
[PDF] |
|
 |
· Roy, K., Zhou, X., Otani, R., Yuan, P., Ioka, S., Vogt, K., Kondo, T., Farag, N., Ijiri, H., Wu, Z., Chitose, Y., Amezawa, M., Uygun, D., Cherasse, Y., Nagase, H., Li, Y., Yanagisawa, M., Abe, M., Basheer, R., Wang, Y.*, Saitoh, T.*, & Lazarus, M.* (2024).
Optochemical control of slow-wave sleep in the nucleus accumbens of male mice by a photoactivatable allosteric modulator of adenosine A2A receptors.
Nature Communications. 15, 3661.
[Full Text]
[PDF] |
|
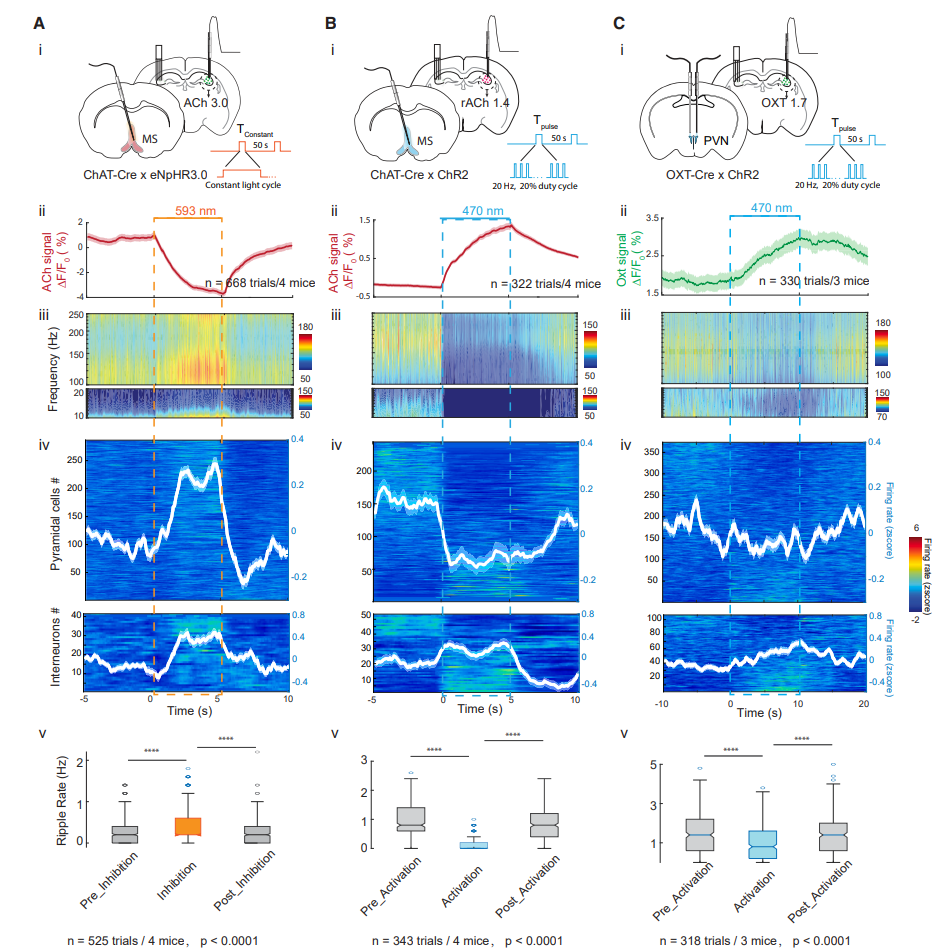 |
· Zhang, Y.#, Karadas, M.#, Liu, J., Gu, X., Vöröslakos, M., Li, Y., Tsien, R. W., & Buzsáki, G.* (2024).
Interaction of acetylcholine and oxytocin neuromodulation in the hippocampus.
Neuron, S0896-6273(24)00154-5.
[Full Text] [PDF] |
|
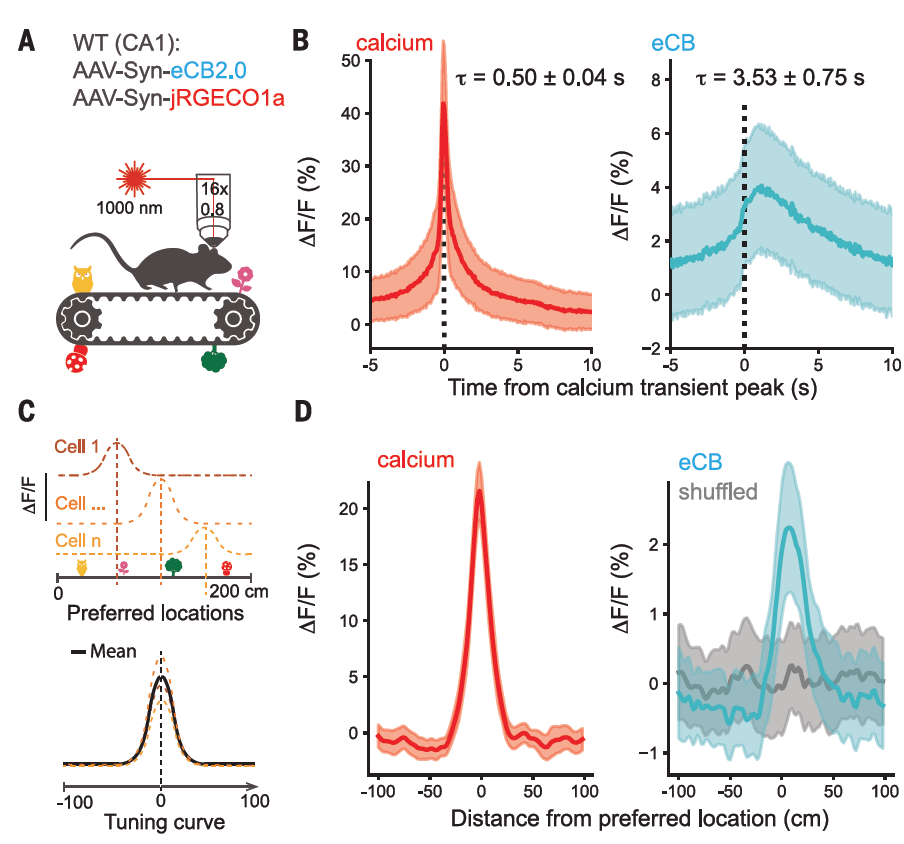 |
· Dudok, B.#*, Fan, L. Z.#, Farrell, J. S., Malhotra, S., Homidan, J., Kim, D. K., Wenardy, C., Ramakrishnan, C., Li, Y., Deisseroth, K., & Soltesz, I.* (2024).
Retrograde endocannabinoid signaling at inhibitory synapses in vivo.
Science, 383(6686), 967-970.
[Full Text] [PDF] |
|
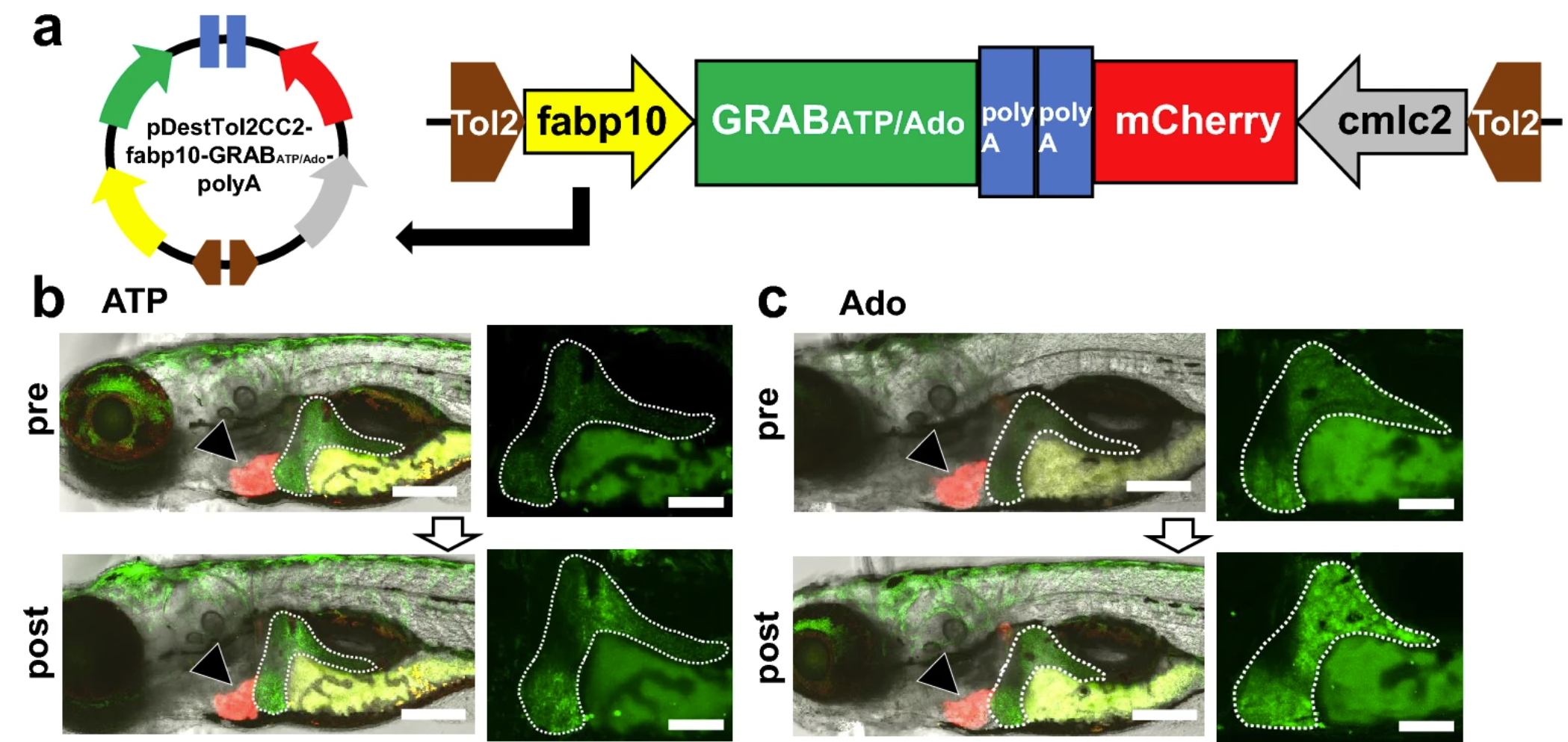 |
· Tokumaru, T., Apolinario, M., Shimizu, N., Umeda, R., Honda, K., Shikano, K., Teranishi, H., Hikida, T., Hanada, T., Ohta, K., Li, Y., Murakami, K., & Hanada, R.* (2024).
Hepatic extracellular ATP/adenosine dynamics in zebrafish models of alcoholic and metabolic steatotic liver disease.
Scientific Reports, 14, 7813.
[Full Text]
[PDF] |
|
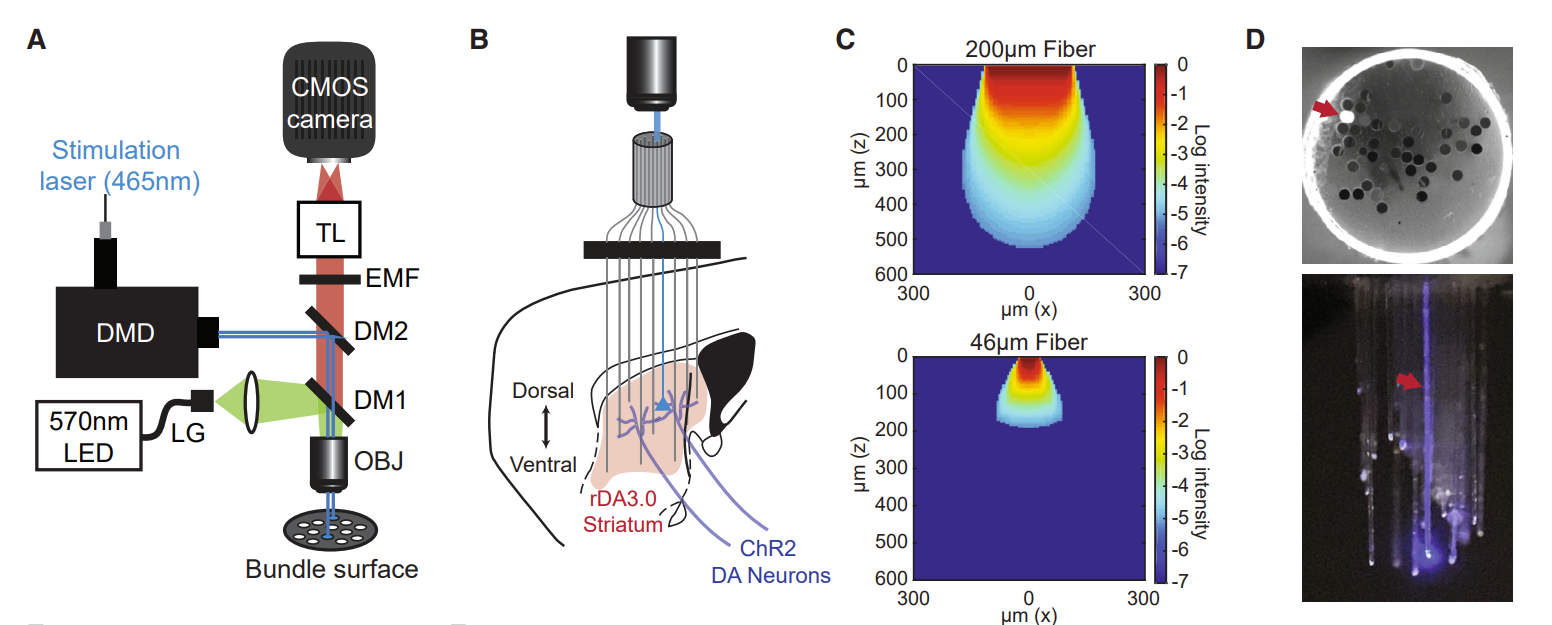 |
· Vu, M., Brown, E., Wen, M., Noggle, C., Zhang, Z,, Monk, K., Bouabid, S., Mroz, L., Graham, B., Zhuo, Y., Li, Y., Otchy, T., Tian, L, Davison, I., Boas, D., & Howe, M.* (2024).
Targeted micro-fiber arrays for measuring and manipulating localized multi-scale neural dynamics over large, deep brain volumes during behavior.
Neuron, Vol 112, Issue 6.
[Full Text]
[PDF] |
|
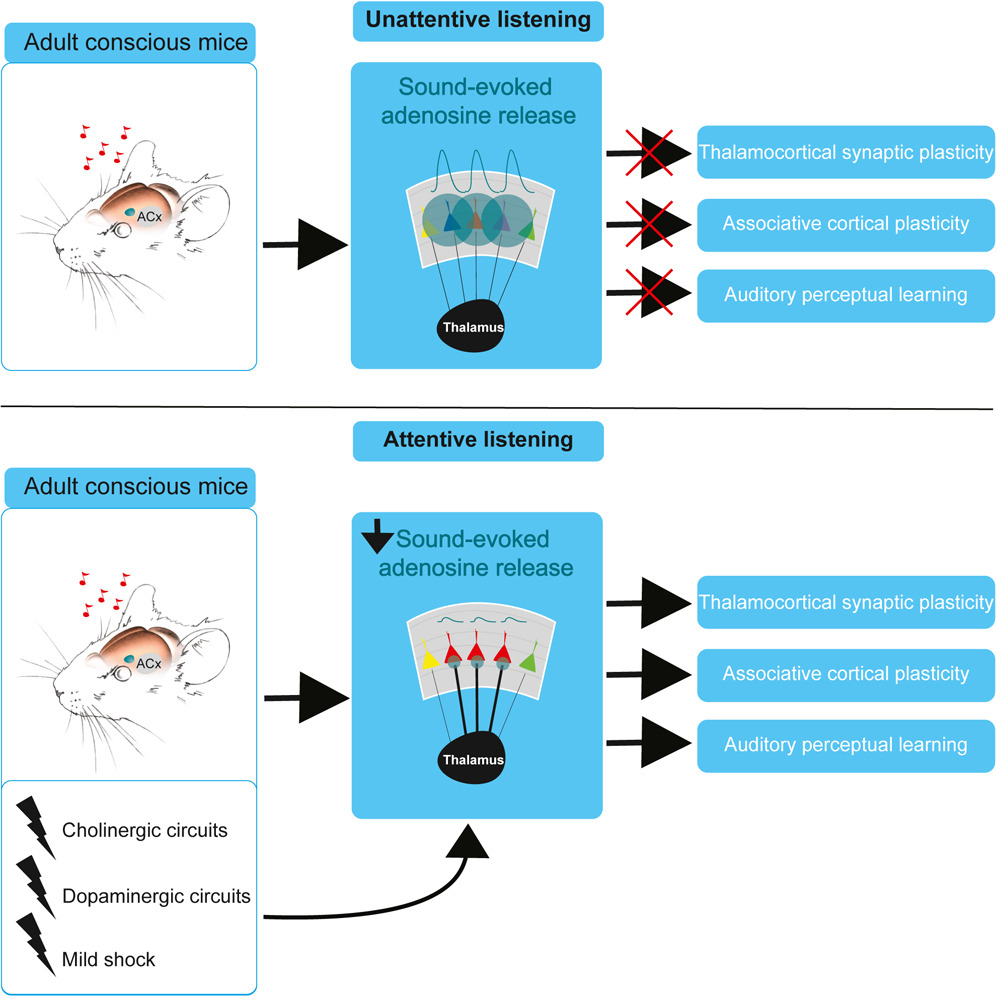 |
· Bayazitov, I., Teubner, B., Feng F., Wu, Z., Li, Y., Blundon, J., & Zakharenko, S.* (2024).
Sound-evoked adenosine release in cooperation with neuromodulatory circuits permits auditory cortical plasticity and perceptual learning.
Cell Reports, Vol 43, Issue 2.
[Full Text]
[PDF] |
|
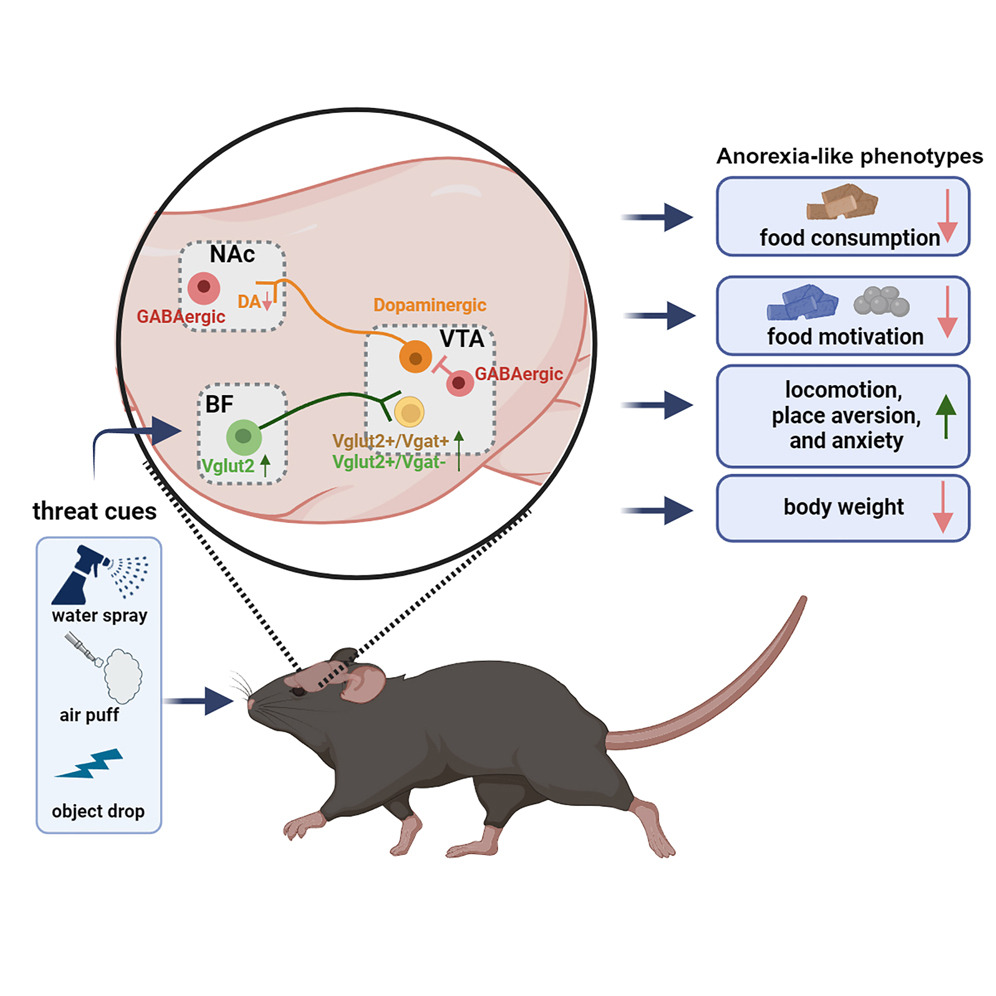 |
· Cai, J., Jiang, Y., Xu, Y., Jiang, Z., Young, C., Hongli L., Ortiz-Guzman, J., Zhuo, Y., Li, Y., Yong X., Arenkiel, B., & Tong, Q.* (2024).
An excitatory projection from the basal forebrain to the ventral tegmental area that underlies anorexia-like phenotypes.
Neuron.. Vol 112, Issue 3.
[Full Text]
[PDF] |
|
 |
· Zhou, X.#, He, Y.#, Xu, T.#, Wu, Z.#, Guo, W., Xu, X., Liu, Y., Zhang, Y., Shang, H., Huang, L., Yao, Z., Li, Z., Su, L., Li, Z., Feng, T., Zhang, S., Monteiro, O., Cunha, R. A., Huang, Z.-L., Zhang, K.*, Li, Y., Cai, X.*, Qu, J.*, & Chen, J.-F.* (2024).
40 Hz light flickering promotes sleep through cortical adenosine signaling.
Cell Research..
[Full Text]
[PDF] |
|
 |
· Gyawali, U., Martin, D. A., Sun, F., Li, Y., & Calu, D.* (2023).
Dopamine in the dorsal bed nucleus of stria terminalis signals Pavlovian sign-tracking and reward violations.
eLife, 12.
[Full Text] [PDF] See also BioRxiv https://doi.org/10.1101/2022.06.21.497039 |
|
 |
· Mayer, F. P.*, Niello, M., Cintulova, D., Sideromenos, S., Maier, J., Li, Y., Bulling, S., Kudlacek, O., Schicker, K., Iwamoto, H., Deng, F., Wan, J., Holy, M., Katamish, R., Sandtner, W., Li, Y., Pollak, D. D., Blakely, R. D., Mihovilovic, M. D., Baumann, M. H., & Sitte, H. H.* (2023).
Serotonin-releasing agents with reduced off-target effects.
Molecular Psychiatry, 28(2), 722-732.
[Full Text] [PDF] See also BioRxiv https://www.researchsquare.com/article/rs-1886596/v1 |
|
 |
· Hatashita, Y., Wu, Z., Fujita, H., Kumamoto, T., Livet, J., Li, Y., Tanifuji, M., & Inoue, T.* (2023).
Spontaneous and multifaceted ATP release from astrocytes at the scale of hundreds of synapses.
Glia , 71(9), 2250-2265.
[Full Text] [PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2022.09.09.507300 |
|
 |
· Singh, S., Sarroza, D., English, A., McGrory, M., Dong, A., Zweifel, L., Land, B. B., Li, Y., Bruchas, M. R., & Stella, N.* (2023).
Pharmacological Characterization of the Endocannabinoid Sensor GRABeCB2.0.
Cannabis and Cannabinoid Research..
[Full Text] [PDF] |
|
 |
· Sang, D.#, Lin, K.#, Yang, Y.#, Ran, G., Li, B., Chen, C., Li, Q., Ma, Y., Lu, L., Cui, X.-Y., Liu, Z., Lv, S.-Q., Luo, M., Liu, Q., Li, Y., & Zhang, E. E.* (2023).
Prolonged sleep deprivation induces a cytokine-storm-like syndrome in mammals.
Cell, 186(25), 5500-5516.e5521.
[Full Text] [PDF] |
|
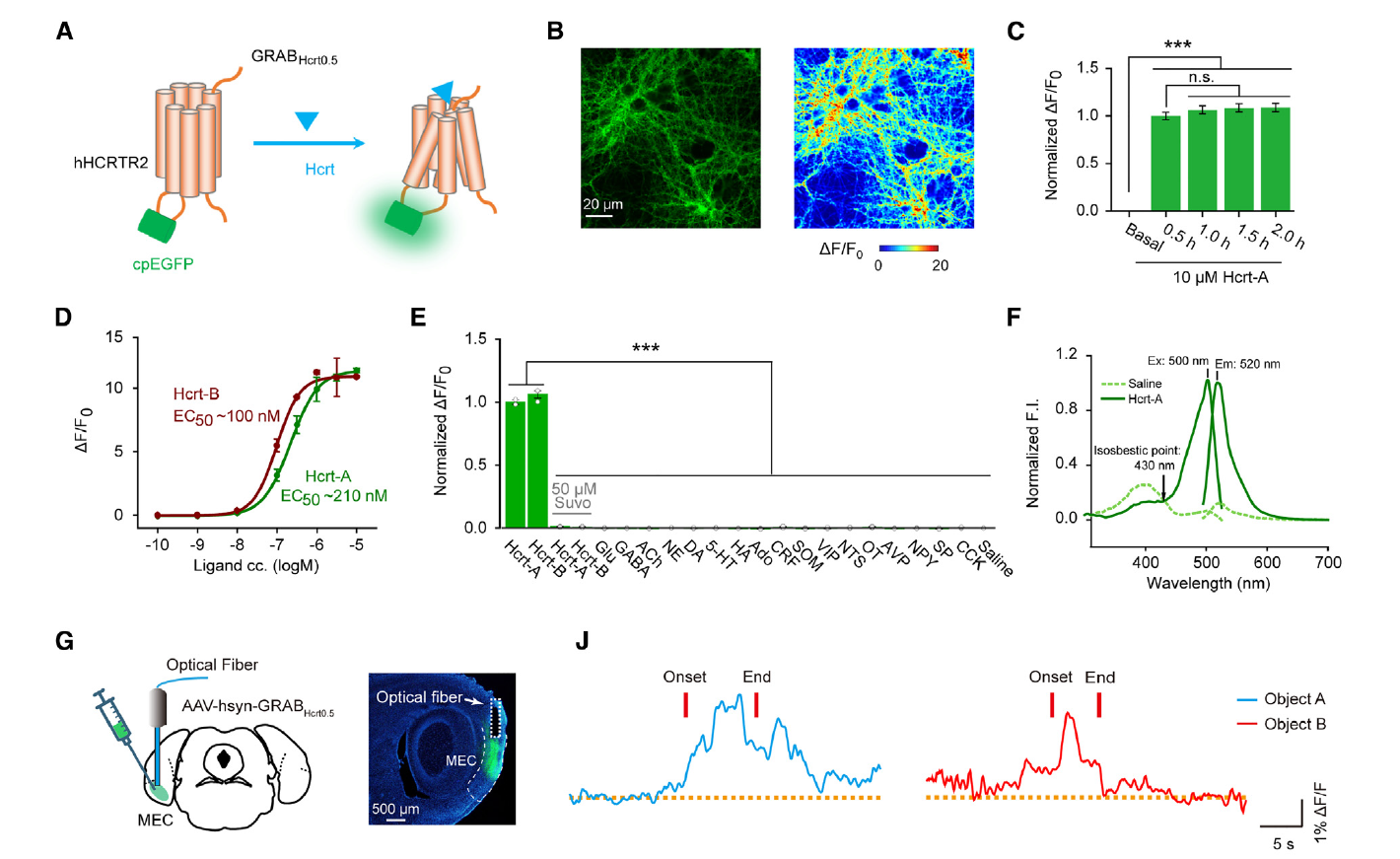 |
· Liao, Y.#, Wen, R.#, Fu, S., Cheng, X., Ren, S., Lu, M., Qian, L., Luo, F., Wang, Y., Xiao, Q., Wang, X., Ye, H., Zhang, X., Jiang, C., Li, X., Li, S., Dang, R., Liu, Y., Kang, J., Yao, Z., Yan, J., Xiong, J., Wang, Y., Wu, S., Chen, X., Li, Y., Xia, J.*, Hu, Z.*, & He, C.* (2023)
Spatial memory requires hypocretins to elevate medial entorhinal gamma oscillations.
Neuron. .
[Full Text] [PDF] |
|
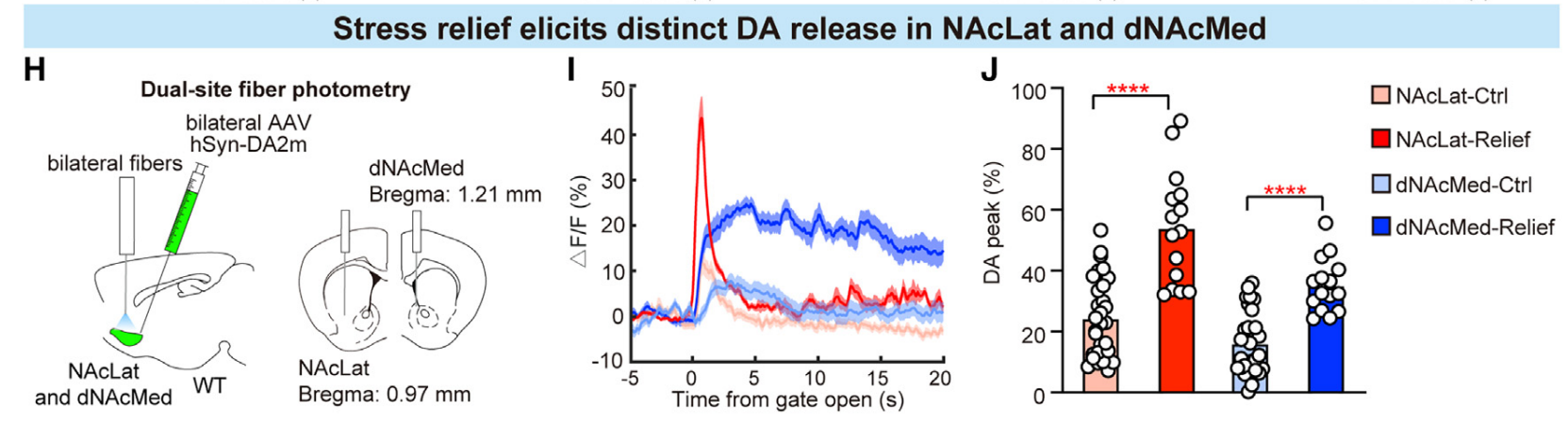 |
· Dong, Y., Li, Y., Xiang, X., Xiao, Z. C., Hu, J., Li, Y., Li, H., & Hu, H.* (2023).
Stress relief as a natural resilience mechanism against depression-like behaviors.
Neuron.
[Full Text] [PDF] |
|
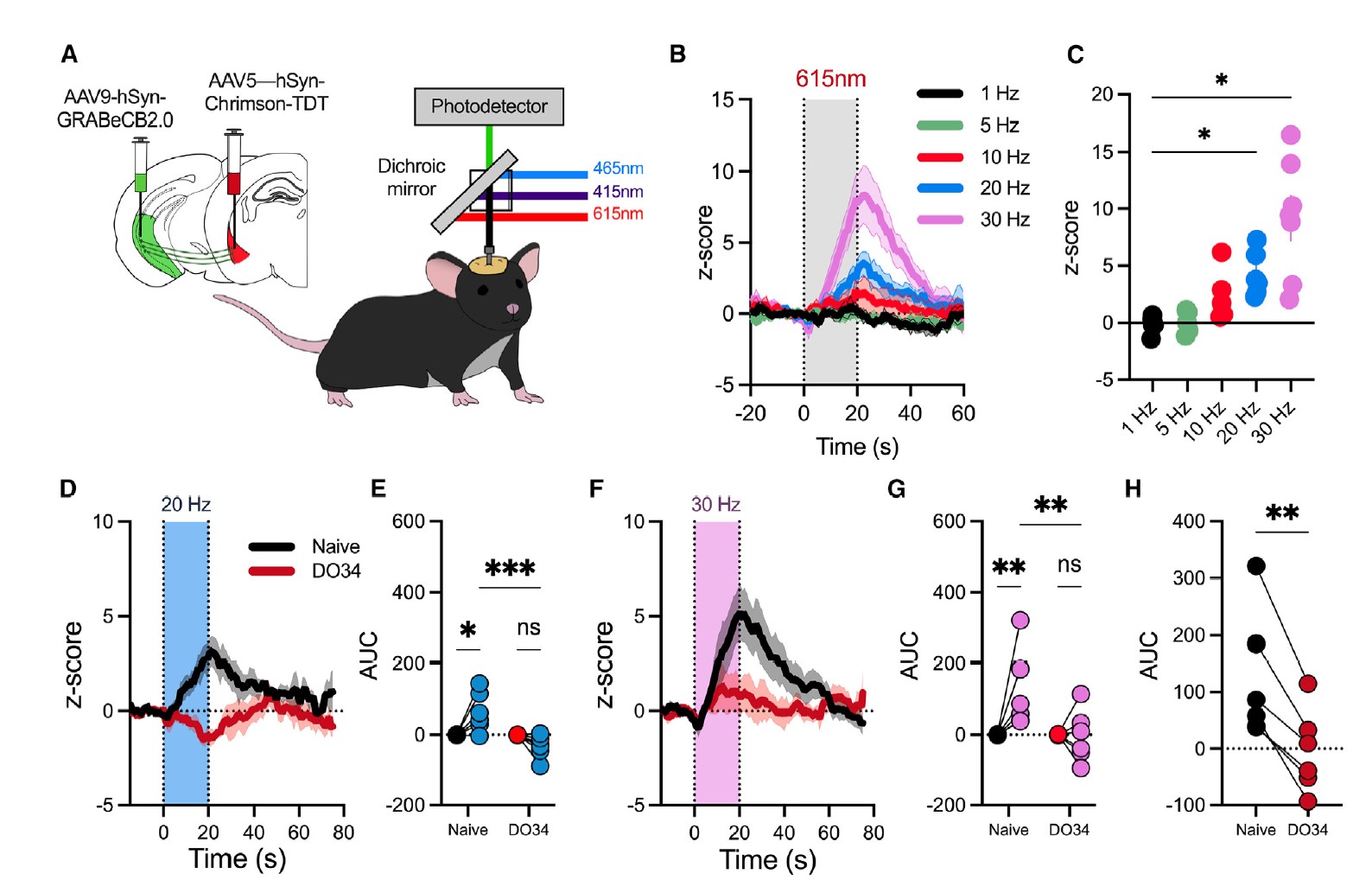 |
· Kondev, V., Najeed, M., Yasmin, F., Morgan, A., Loomba, N., Johnson, K., Adank, D. N., Dong, A., Delpire, E., Li, Y., Winder, D., Grueter, B. A., & Patel, S.* (2023).
Endocannabinoid release at ventral hippocampal-amygdala synapses regulates stress-induced behavioral adaptation.
Cell Reports, 113027.
[Full Text] [PDF] |
|
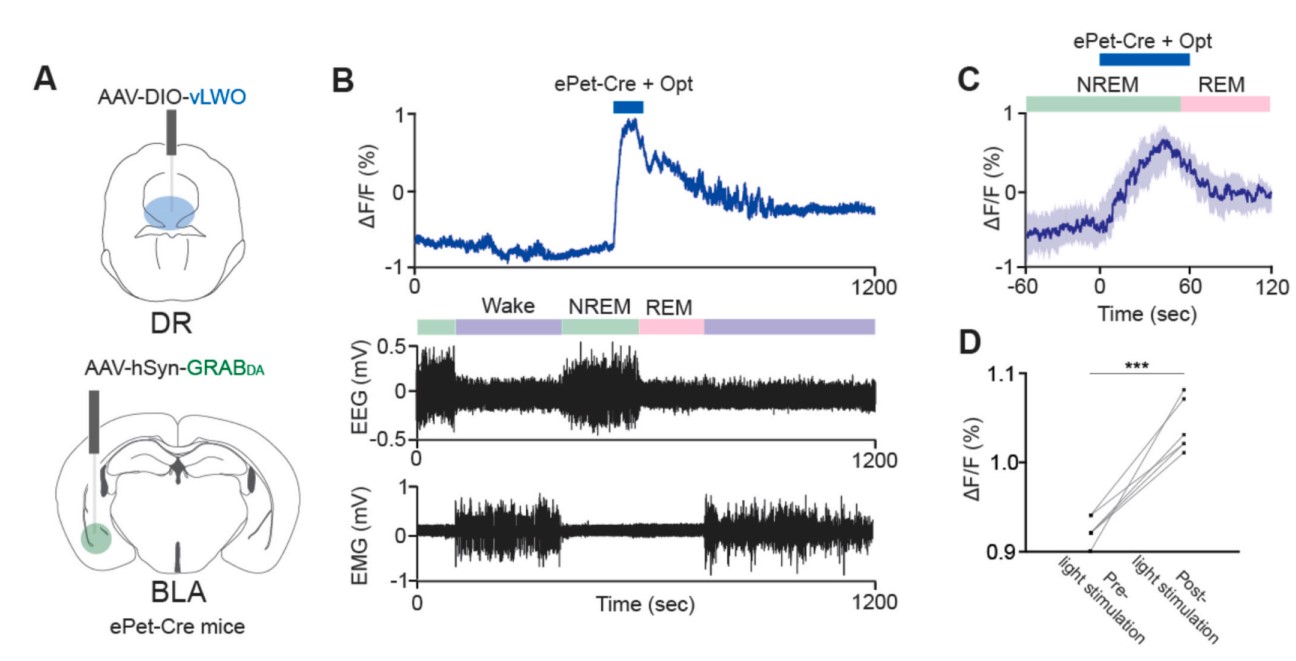 |
· Hasegawa, E., Li, Y., & Sakurai, T.* (2023).
Regulation of REM sleep in mice: The role of dopamine and serotonin function in the basolateral amygdala.
Neuroscience Research.
[Full Text] [PDF] |
|
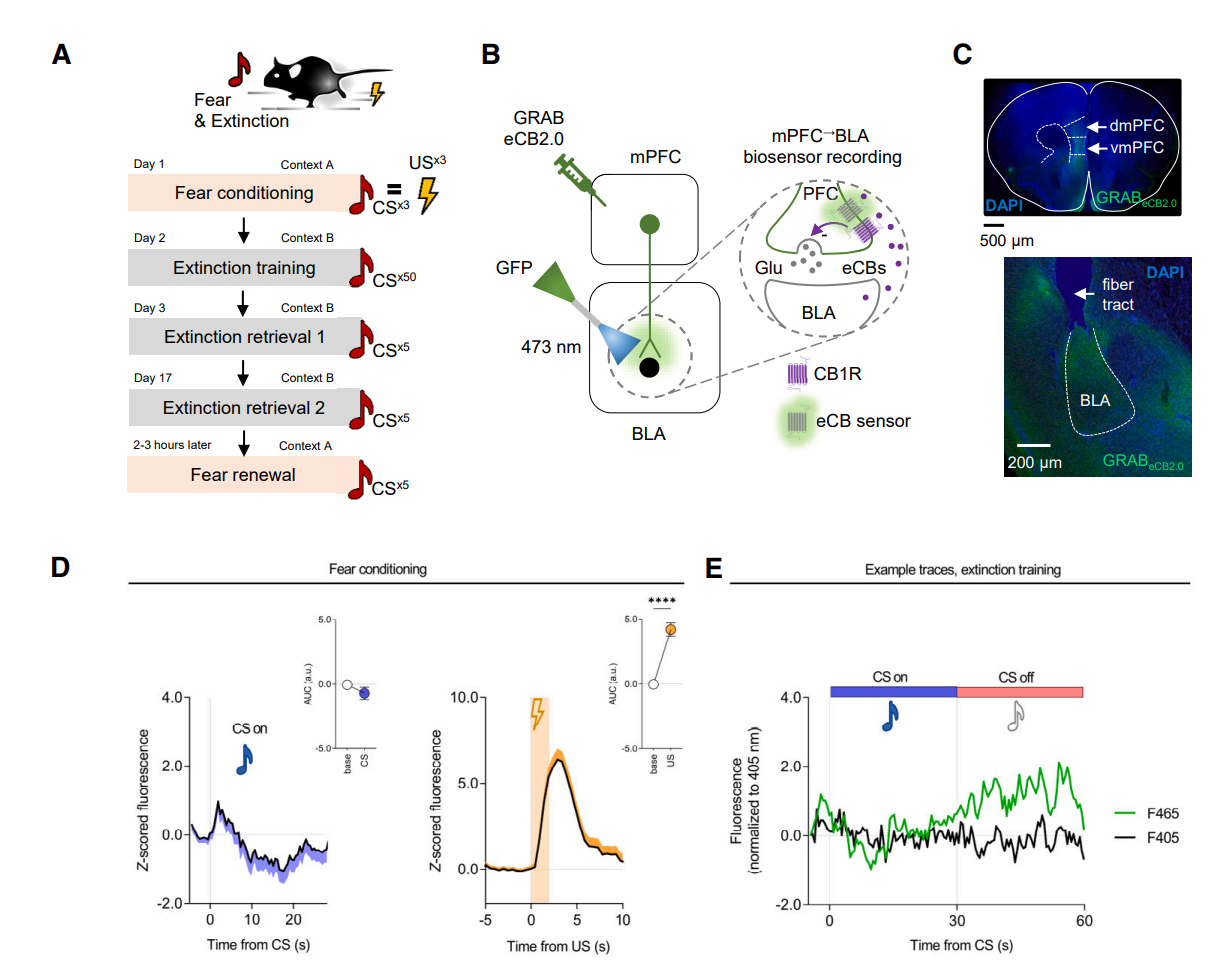 |
· Gunduz-Cinar, O., Castillo, L. I., Xia, M., Van Leer, E., Brockway, E. T., Pollack, G. A., Yasmin, F., Bukalo, O., Limoges, A., Oreizi-Esfahani, S., Kondev, V., Báldi, R., Dong, A., Harvey-White, J., Cinar, R., Kunos, G., Li, Y., Zweifel, L. S., Patel, S., & Holmes, A.* (2023).
A cortico-amygdala neural substrate for endocannabinoid modulation of fear extinction.
Neuron..
[Full Text] [PDF] |
|
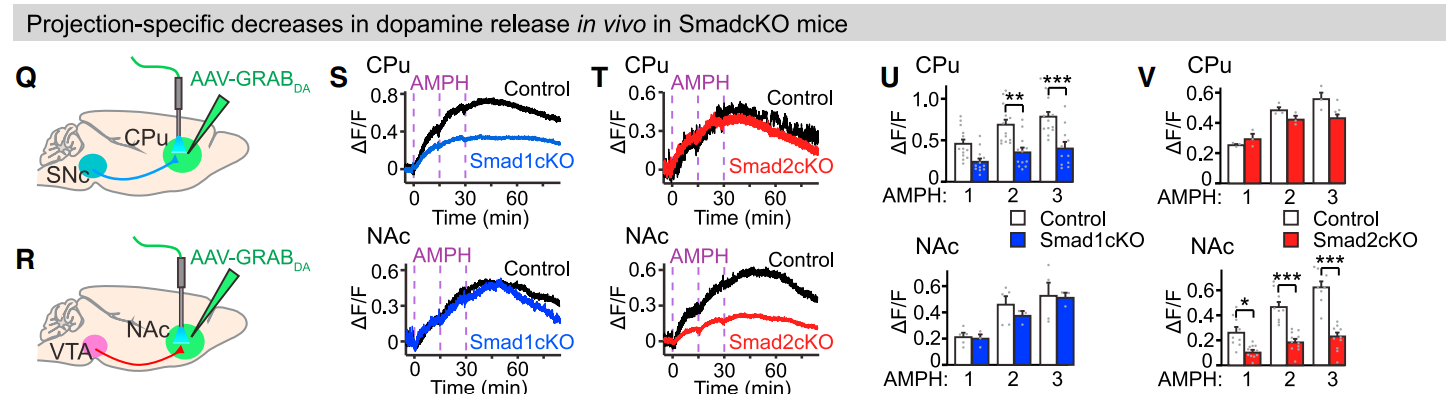 |
· Terauchi, A., Yee, P., Johnson-Venkatesh, E. M., Seiglie, M. P., Kim, L., Pitino, J. C., Kritzer, E., Zhang, Q., Zhou, J., Li, Y., Ginty, D. D., Lee, W. A., & Umemori, H.* (2023).
The projection-specific signals that establish functionally segregated dopaminergic synapses.
Cell.
[Full Text] [PDF] |
|
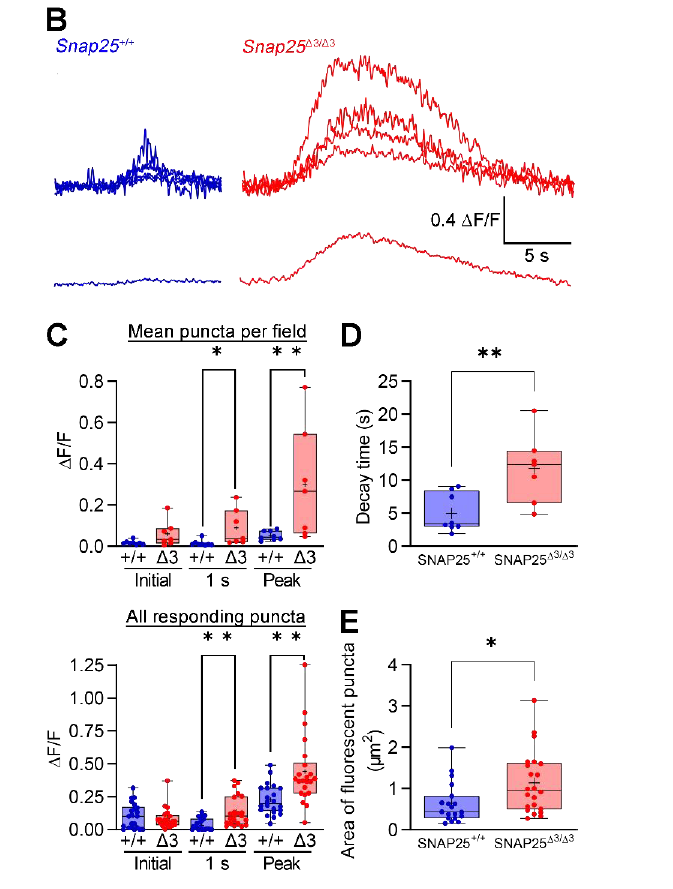 |
· Ceddia, R. P.#, Zurawski, Z.#, Thompson Gray, A., Adegboye, F., McDonald-Boyer, A., Shi, F., Liu, D., Maldonado, J., Feng, J., Li, Y., Alford, S., Ayala, J. E., McGuinness, O. P., Collins, S., & Hamm, H. E.* (2023).
Gβγ-SNAP25 exocytotic brake removal enhances insulin action, promotes adipocyte browning, and protects against diet-induced obesity.
The Journal of Clinical Investigation.
[Full Text] [PDF] |
|
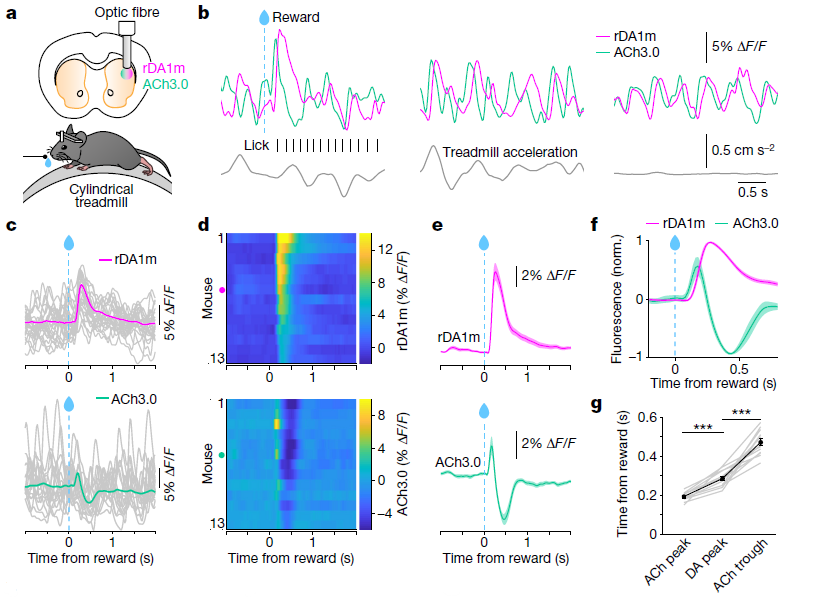 |
· Krok, A. C., Maltese, M., Mistry, P., Miao, X., Li, Y., & Tritsch, N. X.* (2023).
Intrinsic dopamine and acetylcholine dynamics in the striatum of mice.
Nature.
[Full Text] [PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2022.09.09.507300 |
|
 |
· Kimchi, E.*, Burgos-Robles, A., Matthews, G., Chakoma, T., Patarino, M., Weddington, J., Siciliano, C., Yang, W., Foutch, S., Simons, R., Fong, M., Jing, M., Li, Y., Polley, D., Tye, K.* (2023).
Reward contingency gates selective cholinergic suppression of amygdala neurons.
eLife, 12:RP89093
[Full Text] [PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2022.02.04.479188v2 |
|
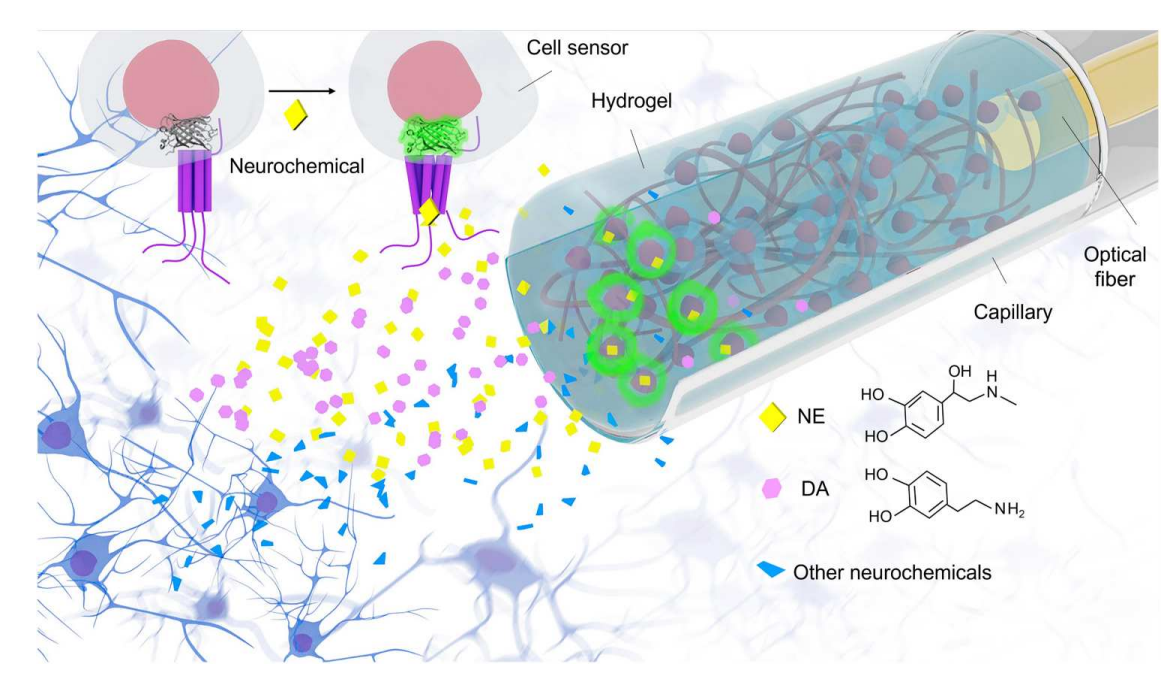 |
· Zhou, B.#, Fan, K.#, Guo, J.#, Feng, J., Yang, C., Li, Y., Shi, S., & Kong, L.* (2023).
Plug-and-play fiber-optic sensors based on engineered cells for neurochemical monitoring at high specificity in freely moving animals.
Science Advances, 9(22), eadg0218.
[Full Text] [PDF] |
|
 |
· Albarran, E., Sun, Y., Liu, Y., Raju, K., Dong, A., Li, Y., Wang, S., Südhof, T. C.*, & Ding, J. B.* (2023).
Postsynaptic synucleins mediate endocannabinoid signaling.
Nature Neuroscience, 26(6), 997-1007.
[Full Text] [PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2021.10.04.462870v1 |
|
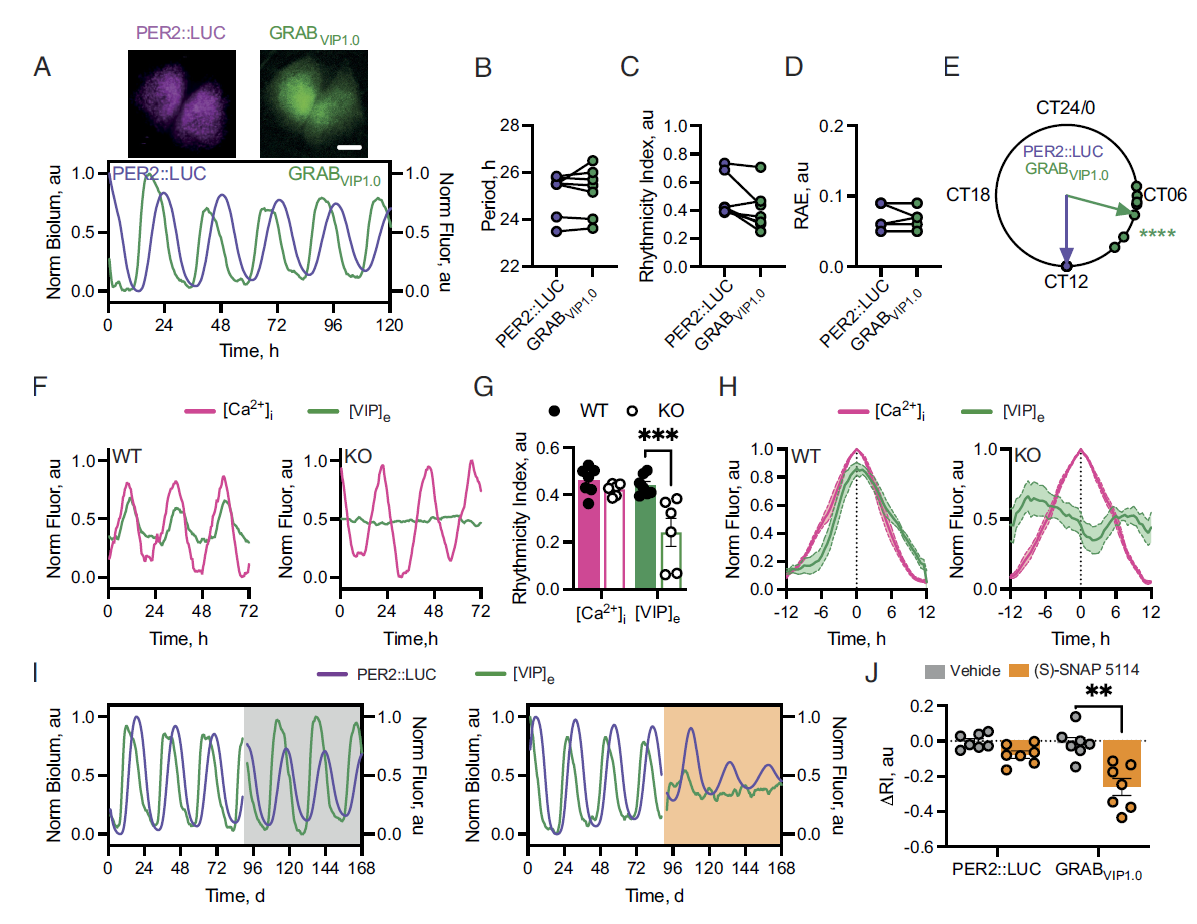 |
· Patton, A. P.*, Morris, E. L., McManus, D., Wang, H., Li, Y., Chin, J. W., & Hastings, M. H.* (2023).
Astrocytic control of extracellular GABA drives circadian timekeeping in the suprachiasmatic nucleus.
Proceedings of the National Academy of Sciences , 120(21), e2301330120.
[Full Text] [PDF] See also BioRxiv https://doi.org/10.1101/2023.01.16.523253 |
|
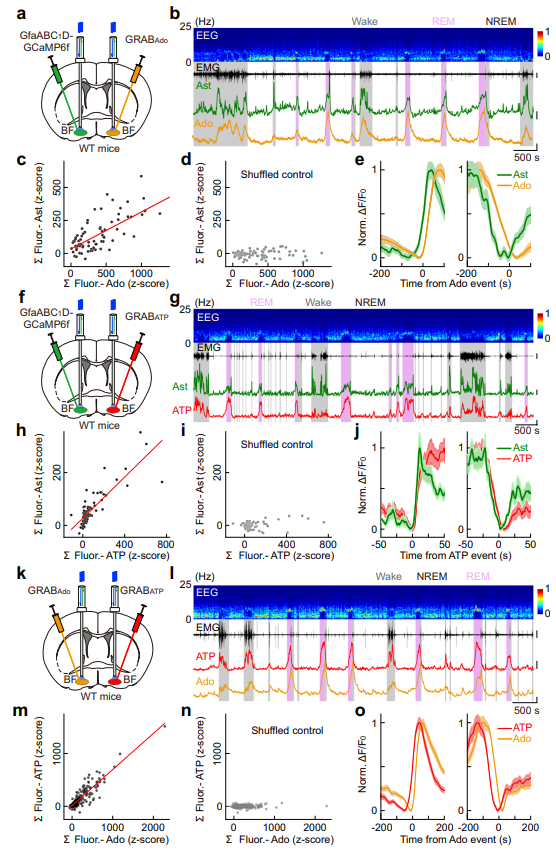 |
· Peng, W.*, Liu, X., Ma, G., Wu, Z., Wang, Z., Fei, X., Qin, M., Wang, L., Li, Y., Zhang, S.*, & Xu, M.* (2023).
Adenosine-independent regulation of the sleep–wake cycle by astrocyte activity.
Cell Discovery, 9(1), 16.
[Full Text] [PDF] |
|
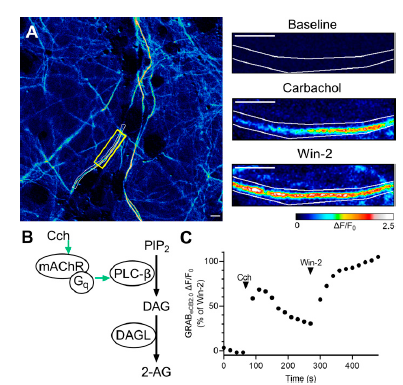 |
· Asher, M. J., McMullan, H. M., Dong, A., Li, Y., & Thayer, S. A.* (2023).
A Complete Endocannabinoid Signaling System Modulates Synaptic Transmission between Human Induced Pluripotent Stem Cell-Derived Neurons.
Mol Pharmacol , 103(2), 100-112.
[Full Text] [PDF] |
|
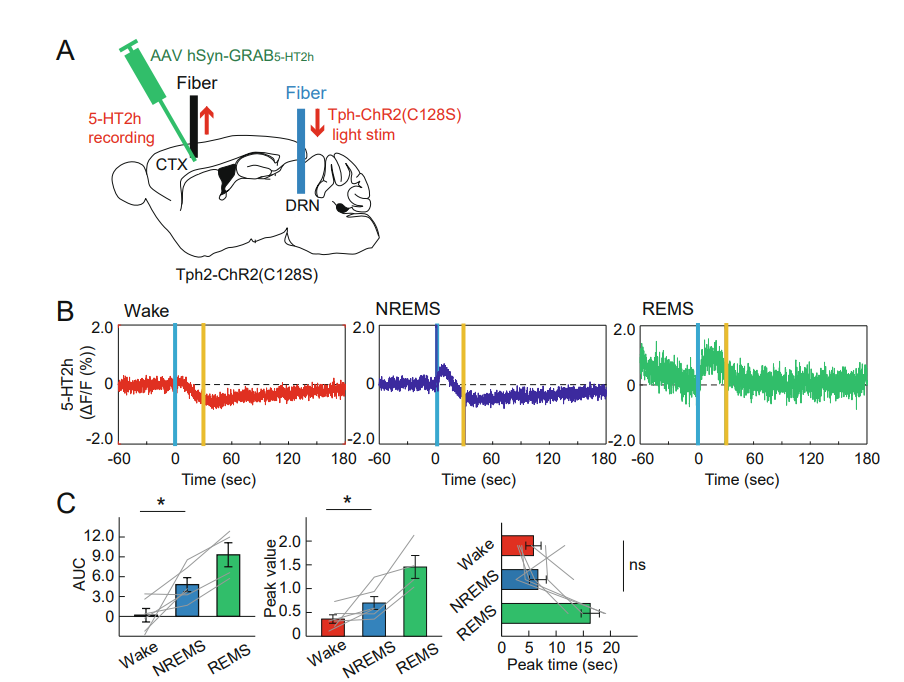 |
· Natsubori, A.*, Hirai, S., Kwon, S., Ono, D., Deng, F., Wan, J., Miyazawa, M., Kojima, T., Okado, H., Karashima, A., Li, Y., Tanaka, K. F., & Honda, M. (2023).
Serotonergic neurons control cortical neuronal intracellular energy dynamics by modulating astrocyte-neuron lactate shuttle.
iScience, 105830.
[Full Text] [PDF] |
|
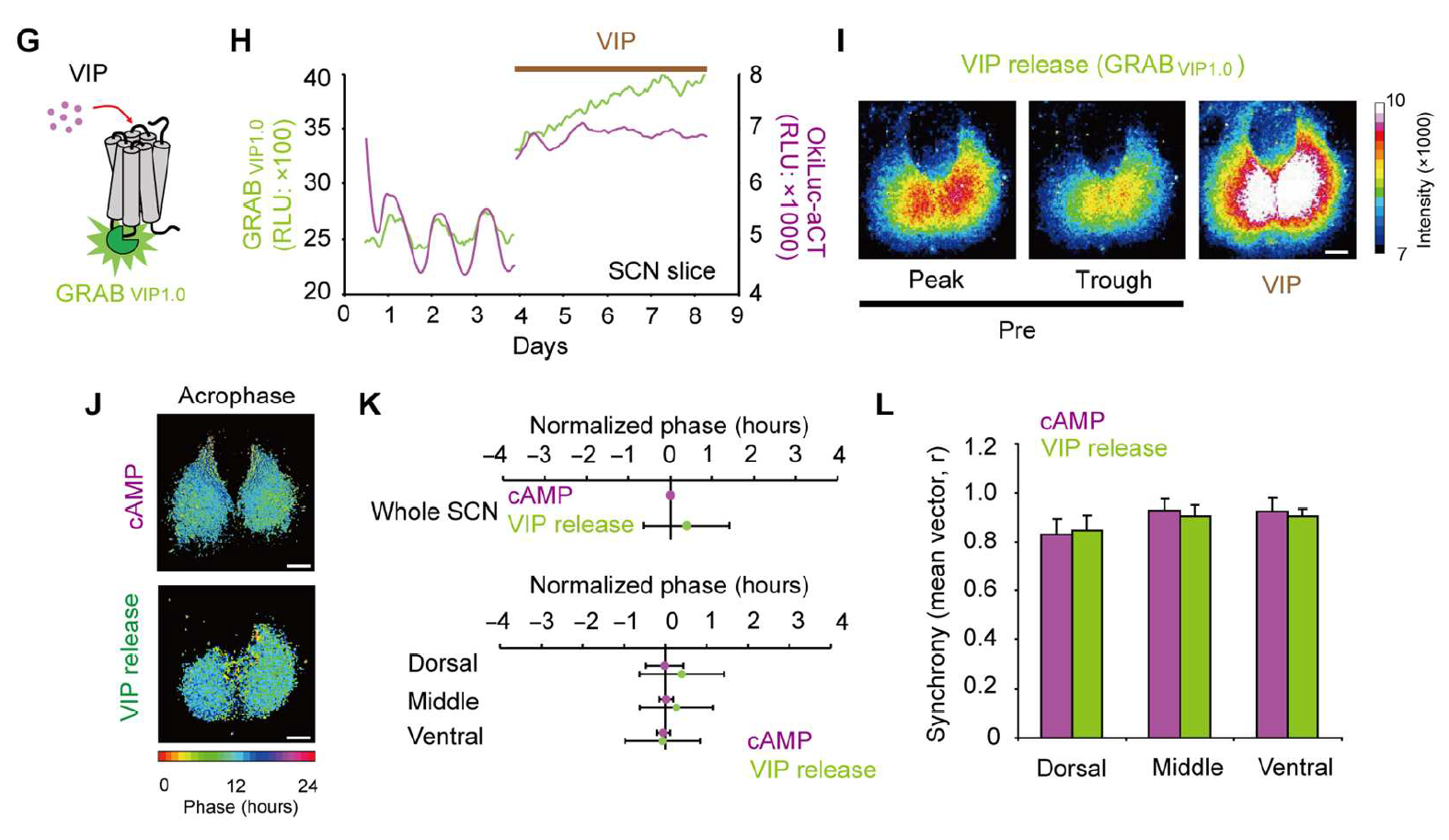 |
· Ono, D.*, Wang, H., Hung, C. J., Wang, H.-t., Kon, N., Yamanaka, A.,Li, Y., & Sugiyama, T. (2023).
Network-driven intracellular cAMP coordinates circadian rhythm in the suprachiasmatic nucleus.
Science Advances, 9(1), eabq7032.
[Full Text] [PDF] |
|
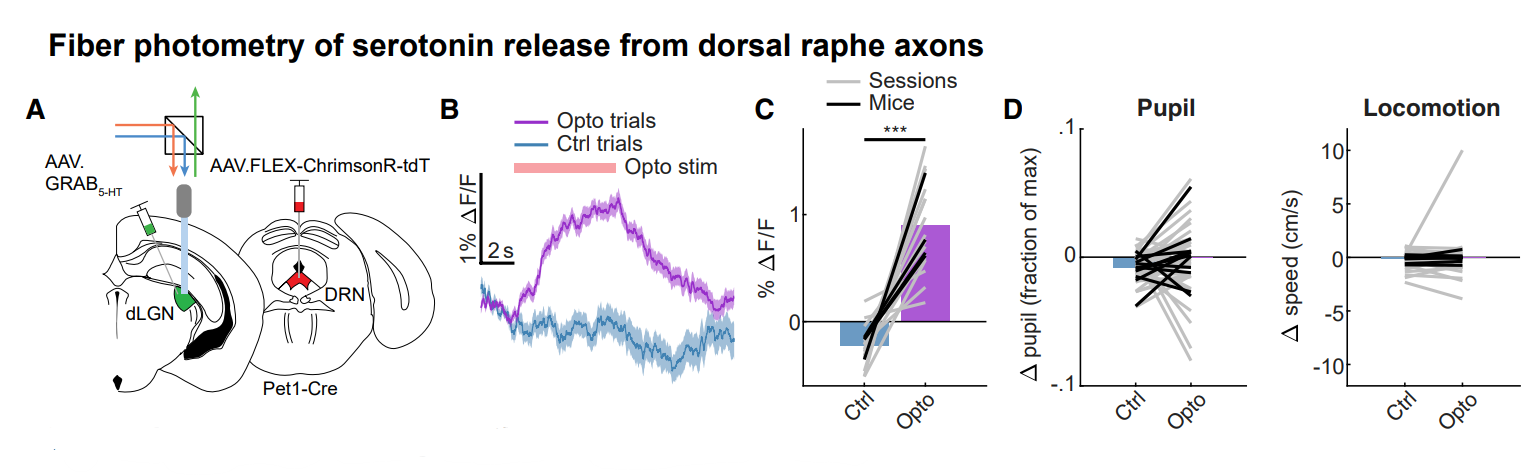 |
· Reggiani, J. D. S., Jiang, Q., Barbini, M., Lutas, A., Liang, L., Fernando, J., Deng, F., Wan, J., Li, Y., Chen, C.*, & Andermann, M. L.* (2022).
Brainstem serotonin neurons selectively gate retinal information flow to thalamus.
Neuron.
[Full Text] [PDF] |
|
 |
· Pittolo, S., Yokoyama, S., Willoughby, D. D., Taylor, C. R., Reitman, M. E., Tse, V., Wu, Z., Etchenique, R., Li, Y., & Poskanzer, K. E.* (2022).
Dopamine activates astrocytes in prefrontal cortex via α1-adrenergic receptors.
Cell Reports, 40(13), 111426.
[Full Text] [PDF] See also BioRxiv https://doi.org/10.1101/2022.07.19.500710 |
|
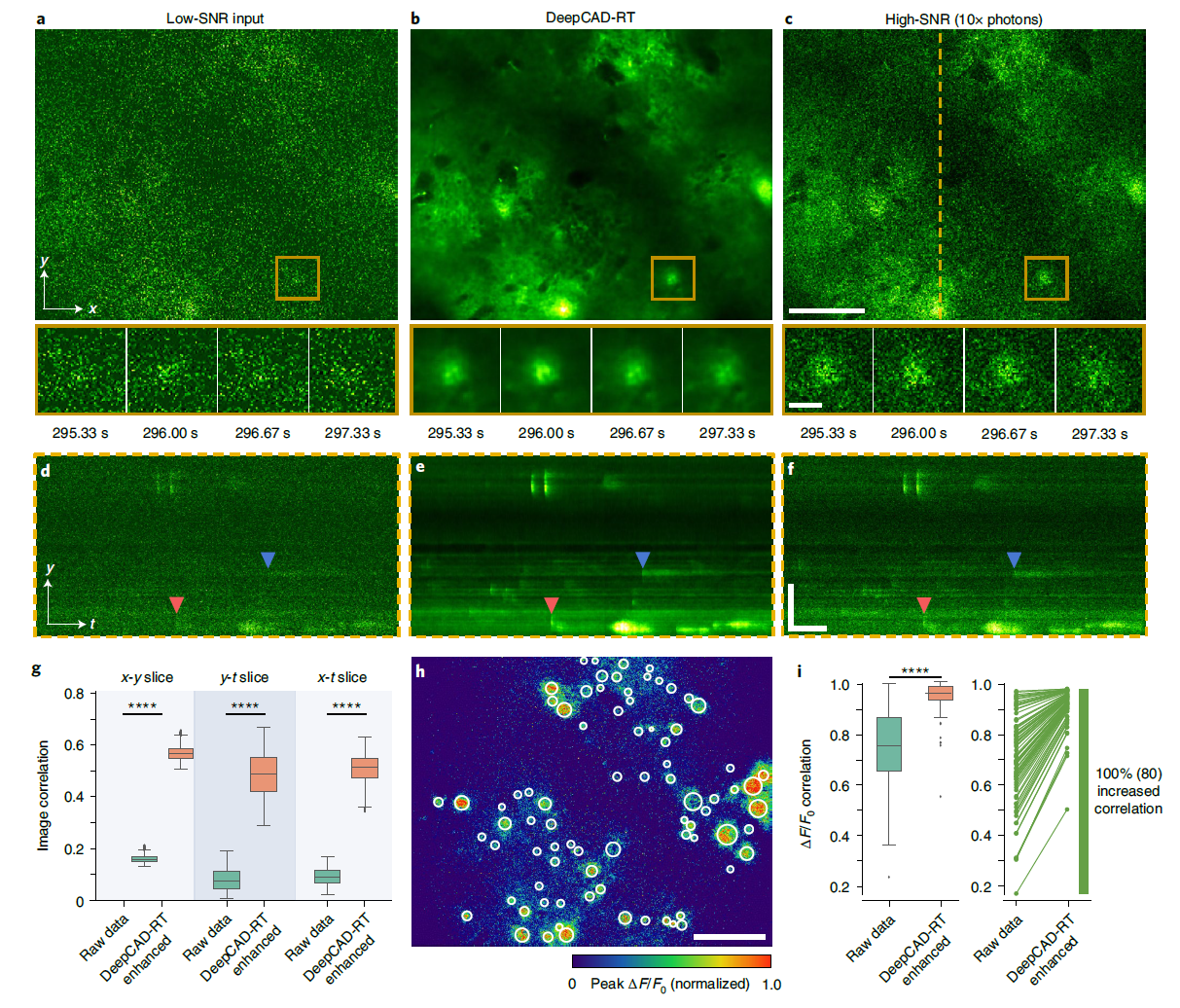 |
· Li, X.#, Li, Y.#, Zhou, Y., Wu, J., Zhao, Z., Fan, J., Deng, F., Wu, Z., Xiao, G., He, J., Zhang, Y., Zhang, G., Hu, X., Chen, X., Zhang, Y., Qiao, H., Xie, H., Li, Y., Wang, H.*, Fang, L.*, & Dai, Q.* (2022).
Real-time denoising enables high-sensitivity fluorescence time-lapse imaging beyond the shot-noise limit.
Nature Biotechnology.
[Full Text] [PDF] See also BioRxiv https://doi.org/10.1101/2022.03.14.484230 |
|
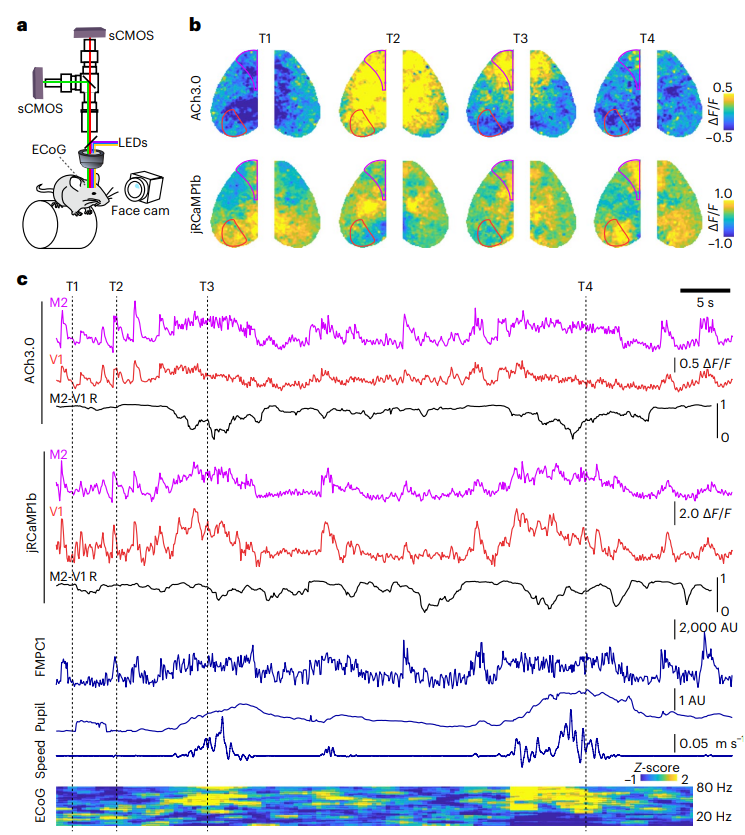 |
· Lohani, S.#, Moberly, A. H.#, Benisty, H., Landa, B., Jing, M., Li, Y., Higley, M. J.*, & Cardin, J. A.* (2022).
Spatiotemporally heterogeneous coordination of cholinergic and neocortical activity. Nature Neuroscience, 25(12), 1706-1713.
[Full Text] [PDF] See also BioRxiv https://doi.org/10.1101/2020.12.09.418632 |
|
 |
· Wang, L.#, Wu, C.#, Peng, W., Zhou, Z., Zeng, J., Li, X., Yang, Y., Yu, S., Zou, Y., Huang, M., Liu, C., Chen, Y., Li, Y., Ti, P., Liu, W., Gao, Y., Zheng, W., Zhong, H., Gao, S., Lu, Z., Ren, P.-G., Ng, H. L., He, J., Chen, S., Xu, M., Li, Y., & Chu, J.* (2022).
A high-performance genetically encoded fluorescent indicator for in vivo cAMP imaging.
Nature Communications, 13(1), 5363.
[Full Text] [PDF] See also BioRxiv https://doi.org/10.1101/2022.02.27.482140 |
|
 |
· Sheu, S.-H.*, Upadhyayula, S., Dupuy, V., Pang, S., Deng, F., Wan, J., Walpita, D., Pasolli, H. A., Houser, J., Sanchez-Martinez, S., Brauchi, S. E., Banala, S., Freeman, M., Xu, C. S., Kirchhausen, T., Hess, H. F., Lavis, L., Li, Y., Chaumont-Dubel, S., & Clapham, D. E.* (2022).
A serotonergic axon-cilium synapse drives nuclear signaling to alter chromatin accessibility. Cell, 185(18), 3390-3407.e3318.
[Full Text] [PDF] See also BioRxiv https://doi.org/10.1101/2021.09.27.461878 * See Preview by: Simon, D. J., & Levitz, J. (2022).
A ciliary synapse for “short-circuit” neuromodulation.
Cell , 185(18), 3284-3286.
[Full Text] [PDF] |
|
 |
· Dai, B.*, Sun, F., Tong, X., Ding, Y., Kuang, A., Osakada, T., Li, Y., & Lin, D.* (2022).
Responses and functions of dopamine in nucleus accumbens core during social behaviors. Cell Reports, 40(8), 111246.
[Full Text] [PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2021.06.22.449478v1 |
|
 |
· Lin, R.*#, Zhou, Y.#, Yan, T.#, Wang, R.#, Li, H., Wu, Z., Zhang, X., Zhou, X., Zhao, F., Zhang, L., Li, Y., & Luo, M.* (2022).
Directed evolution of adeno-associated virus for efficient gene delivery to microglia. Nat Methods, 19(8), 976-985.
[Full Text] [PDF] |
|
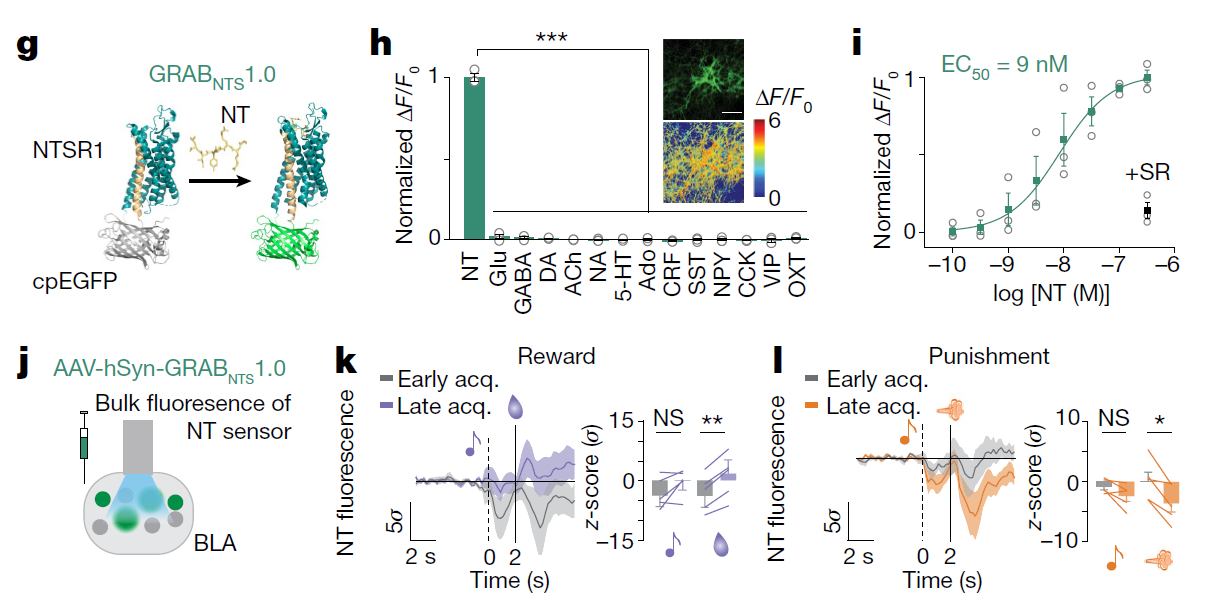 |
· Li, H.#, Namburi, P.#, Olson, J. M.#, Borio, M., Lemieux, M. E., Beyeler, A., Calhoon, G. G., Hitora-Imamura, N., Coley, A. A., Libster, A., Bal, A., Jin, X., Wang, H., Jia, C., Choudhury, S. R., Shi, X., Felix-Ortiz, A. C., de la Fuente, V., Barth, V. P., King, H. O., Izadmehr, E. M., Revanna, J. S., Batra, K., Fischer, K. B., Keyes, L. R., Padilla-Coreano, N., Siciliano, C. A., McCullough, K. M., Wichmann, R., Ressler, K. J., Fiete, I. R., Zhang, F., Li, Y., & Tye, K. M.* (2022).
Neurotensin orchestrates valence assignment in the amygdala. Nature.
[Full Text] [PDF] |
|
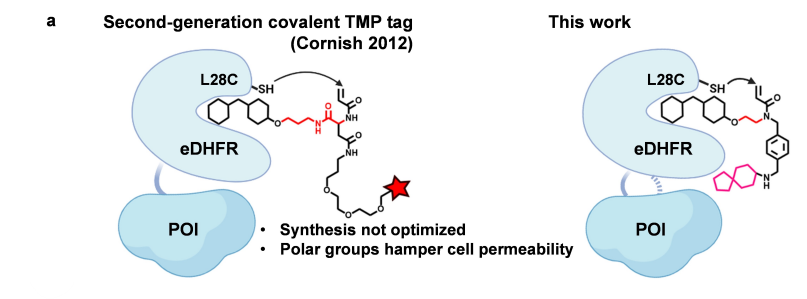 |
· Mo, J.#, Chen, J.#, Shi, Y., Sun, J., Wu, Y., Liu, T., Zhang, J., Zheng, Y., Li, Y., & Chen, Z.* (2022).
Third-Generation Covalent TMP-Tag for Fast Labeling and Multiplexed Imaging of Cellular Proteins Angewandte Chemie International Edition, e202207905.
[Full Text] [PDF] |
|
 |
· Yu, X.#*, Zhao, G.#, Wang, D., Wang, S., Li, R., Li, A., Wang, H., Nollet, M., Chun, Y. Y., Zhao, T., Yustos, R., Li, H., Zhao, J., Li, J., Cai, M., Vyssotski, A. L., ,Li, Y., Dong, H.*, Franks, N. P.*, & Wisden, W.* (2022).
A specific circuit in the midbrain detects stress and induces restorative sleep. Science, 377(6601), 63-72. [Full Text] [PDF] |
|
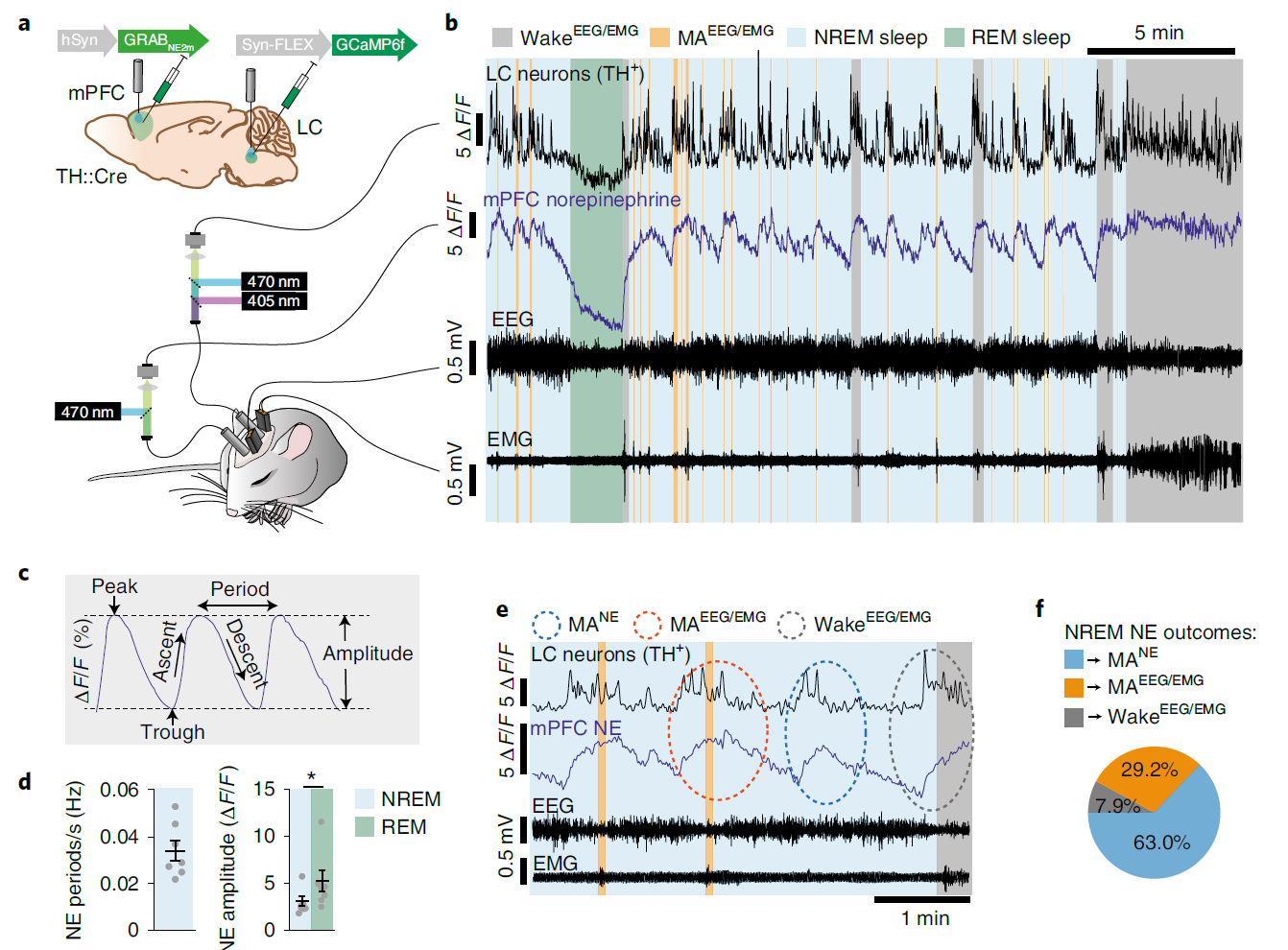 |
· Kjaerby, C.*, Andersen, M., Hauglund, N., Untiet, V., Dall, C., Sigurdsson, B., Ding, F., Feng, J., Li, Y., Weikop, P., Hirase, H., & Nedergaard, M.* (2022).
Memory-enhancing properties of sleep depend on the oscillatory amplitude of norepinephrine. Nature Neuroscience.
[Full Text] [PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2020.09.01.274977v1 * See Comments Highlight by: Morici, J. F., & Girardeau, G.* (2022).
Cortical norepinephrine GRABs a seat at the sleep table.
Nature Neuroscience, 25(8), 978-980.
[Full Text] [PDF] |
|
 |
· Han, J., Yoon, J., Shin, J., Nam, E., Qian, T.,Li, Y., Park, K.*, Lee, S.-H.*, & Lim, M. H.* (2022).
Conformational and functional changes of the native neuropeptide somatostatin occur in the presence of copper and amyloid-β. Nature Chemistry. [Full Text] [PDF] See also ChemRxiv https://doi.org/10.26434/chemrxiv.14736882 |
|
 |
· Klein Herenbrink, C.#, Støier, J. F.#, Reith, W. D., Dagra, A., Gregorek, M. A. C., Cola, R. B., Patriarchi, T., Li, Y., Tian, L., Gether, U., & Herborg, F.* (2022). Multimodal detection of dopamine by sniffer cells expressing genetically encoded fluorescent sensors. Communications Biology, 5(1), 578.
[Full Text] [PDF] See also BioRxiv https://doi.org/10.1101/2021.09.16.460471 |
|
 |
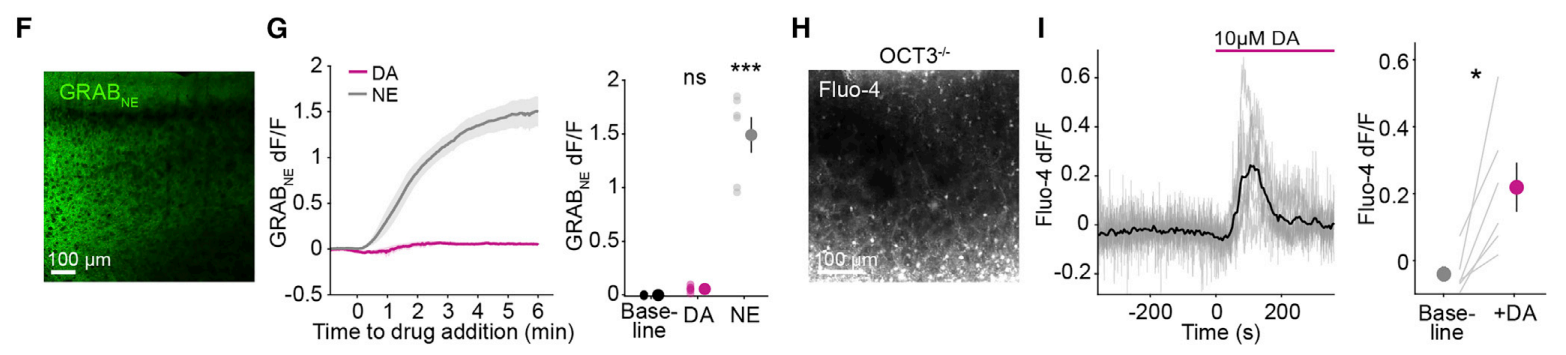
· Breton-Provencher, V.#*, Drummond, G. T.#, Feng, J., Li, Y., & Sur, M.* (2022).
Spatiotemporal dynamics of noradrenaline during learned behaviour. Nature [Full Text] [PDF] |
|
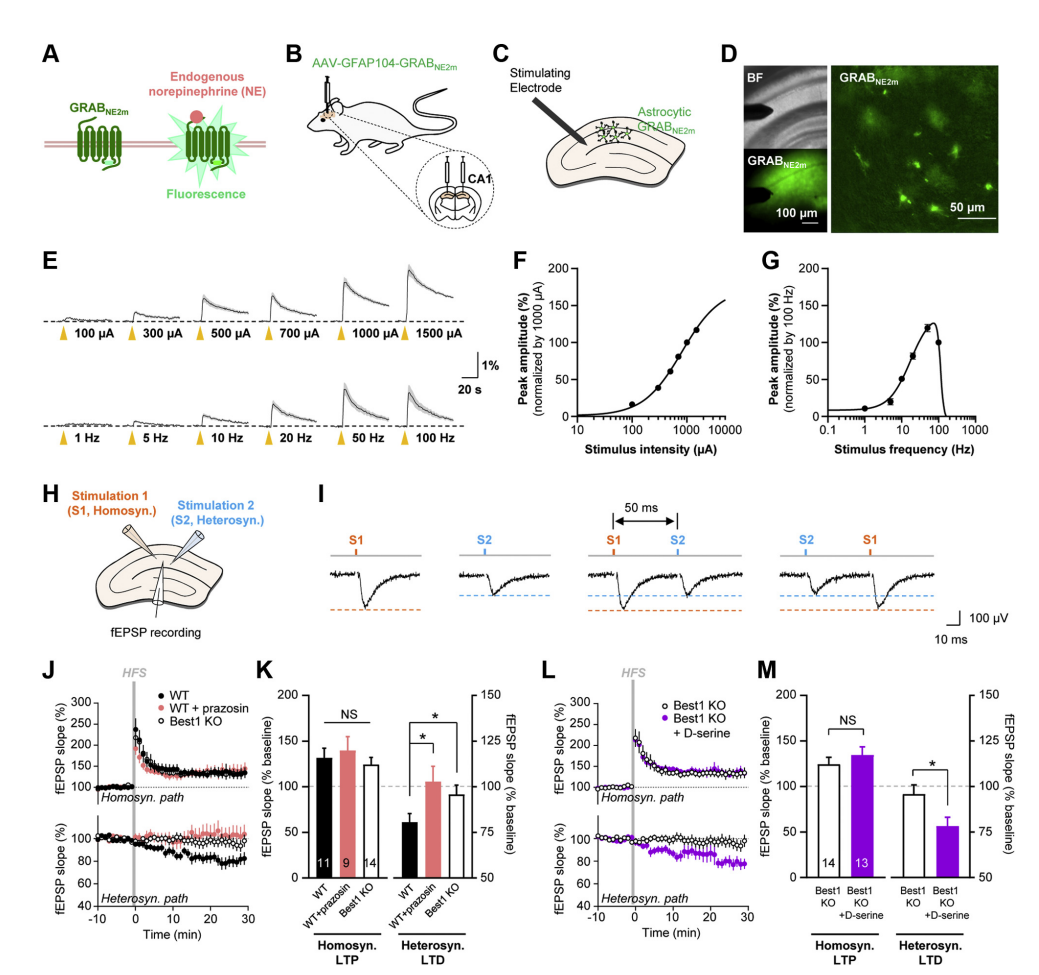 |
· Koh, W., Park, M., Chun, Y. E., Lee, J., Shim, H. S., Park, M. G., Kim, S., Sa, M., Joo, J., Kang, H., Oh, S.-J., Woo, J., Chun, H., Lee, S. E., Hong, J., Feng, J., Li, Y., Ryu, H., Cho, J., & Lee, C. J.* (2021).
Astrocytes Render Memory Flexible by Releasing D-Serine and Regulating NMDA Receptor Tone in the Hippocampus.
Biological Psychiatry, 91(8), 740-752. [Full Text] [PDF] See also BioRxiv https://doi.org/10.1101/2021.03.25.436945 |
|
 |
· Stahl, A., Noyes, N. C., Boto, T., Botero, V., Broyles, C. N., Jing, M., Zeng, J., King, L. B., Li, Y., Davis, R. L., & Tomchik, S. M.* (2022). Associative learning drives longitudinally graded presynaptic plasticity of neurotransmitter release along axonal compartments. eLife, 11.
[Full Text] [PDF] See also BioRxiv https://doi.org/10.1101/2021.06.08.447536 |
|
 |
· Liu, C.*, Cai, X., Ritzau-Jost, A., Kramer Paul, F., Li, Y.., Khaliq Zayd, M., Hallermann, S., & Kaeser Pascal, S.* (2022).
An action potential initiation mechanism in distal axons for the control of dopamine release. Science, 375(6587), 1378-1385. [Full Text] [PDF] * See Comments Highlight by: Wiseman, S. (2022).
Dopamine surprises.
Nature Neuroscience, 25(5), 531-531.
[Full Text] [PDF] |
|
.png) |
· Hasegawa, E., Miyasaka, A., Sakurai, K., Cherasse, Y., Li, Y.., & Sakurai, T.* (2022) Rapid eye movement sleep is initiated by basolateral amygdala dopamine signaling in mice.
Science, 375(6584), 994-1000. [Full Text] [PDF] |
|
 |
· Liput, D. J., Puhl, H. L., Dong, A., He, K., Li, Y., & Lovinger, D. M. (2022).
2-Arachidonoylglycerol mobilization following brief synaptic stimulation in the dorsal lateral striatum requires glutamatergic and cholinergic neurotransmission. Neuropharmacology, 205, 108916. [Full Text] [PDF] |
|
 |
· Deng, H.*, Xiao, X., Yang, T., Ritola, K., Hantman, A., Li, Y., Huang, Z. J., & Li, B.* (2021).
A genetically defined insula-brainstem circuit selectively controls motivational vigor.
Cell, 184(26), 6344-6360.e6318. [Full Text] [PDF] |
|
 |
· Hamilos, A. E., Spedicato, G., Hong, Y., Sun, F., Li, Y., & Assad, J. (2021). Slowly evolving dopaminergic activity modulates the moment-to-moment probability of reward-related self-timed movements.
eLife,, 10, e62583. [Full Text] [PDF] |
|
 |
· Lu, C.-L.#, Ren, J.#, Mo, J.-W., Fan, J., Guo, F., Chen, L.-Y., Wen, Y.-L., Li, S.-J., Fang, Y.-Y., Wu, Z.-F., Li, Y., Gao, T.-M., & Cao, X.*(2021)
Glucocorticoid receptor-dependent astrocytes mediate stress vulnerability. Biological Psychiatry, 12(1), 6403. [Full Text] [PDF] |
|
 |
· Gallo, E. F.*, Greenwald, J., Yeisley, J., Teboul, E., Martyniuk, K. M., Villarin, Jing, M., Li, Y., Javitch, J. A., Balsam, P. D., & Kellendonk, C*. (2022).
Dopamine D2 receptors modulate the cholinergic pause and inhibitory learning. Mol Psychiatry, 27(3), 1502-1514. [Full Text] [PDF] See also BioRxiv https://www.biorxiv.org/content/10.1101/2020.09.07.284612 |
|
 |
· Robert, B., Kimchi, E. Y., Watanabe, Y., Chakoma, T., Jing, M., Li, Y., & Polley, D. B. (2021). A functional topography within the cholinergic basal forebrain for encoding sensory cues and behavioral reinforcement outcomes. eLife, 10, e69514.
[Full Text] [PDF] |
|
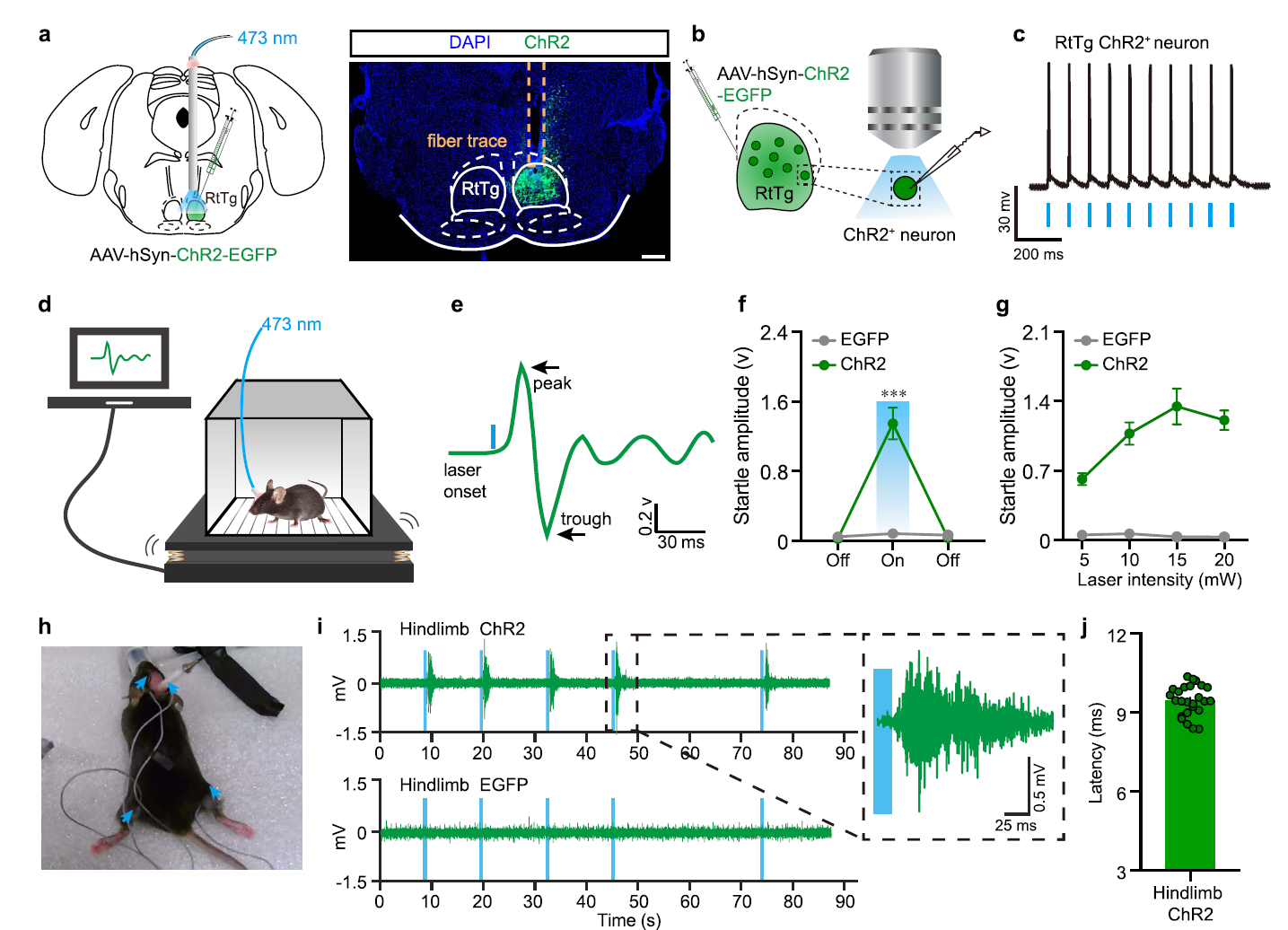 |
· Guo, W.#, Fan, S.#, Xiao, D., Dong, H., Xu, G., Wan, Z., Ma, Y., Wang, Z., Xue, T., Zhou, Y., Li, Y., & Xiong, W.* (2021).
A Brainstem reticulotegmental neural ensemble drives acoustic startle reflexes. Nature Communications, 12(1), 6403. [Full Text] [PDF] |
|
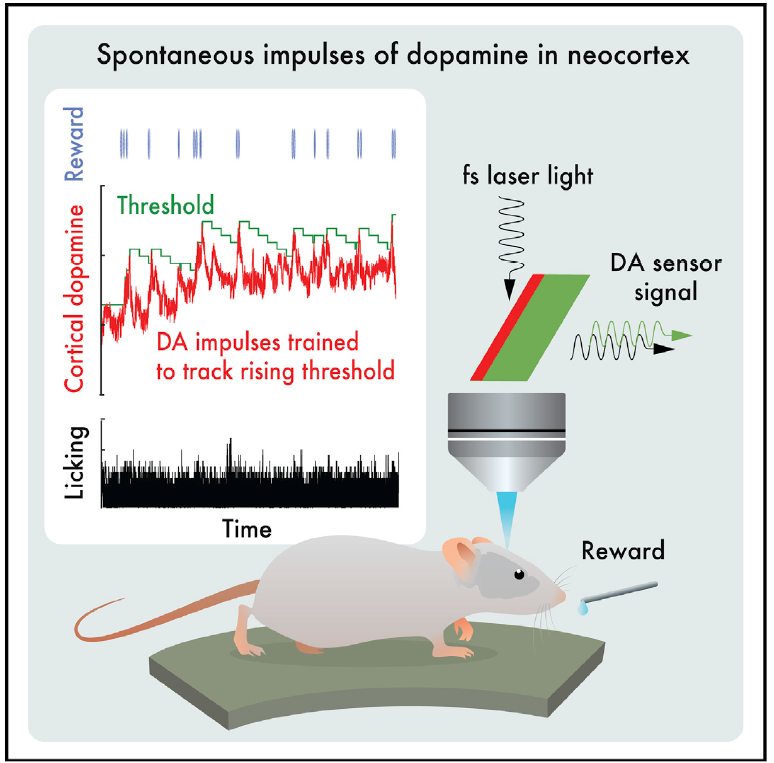 |
· Foo, C., Lozada, A., Aljadeff, J., Li, Y., Wang, J. W., Slesinger, P. A.*, & Kleinfeld, D.* (2021). Reinforcement learning links spontaneous cortical dopamine impulses to reward. Current Biology, 31(18), 4111-4119.e4114.
[Full Text] [PDF] |
|
 |
· Li, Y., Simmler Linda, D., Van Zessen, R., Flakowski, J., Wan, J.-X., Deng, F., Li, Y.-L., Nautiyal Katherine, M., Pascoli, V., & Lüscher, C.* (2021)
Synaptic mechanism underlying serotonin modulation of transition to cocaine addiction. Science, 73(6560), 1252-1256. [Full Text] [PDF] |
|
 |
· Al-Hasani, R.#*, Gowrishankar, R.#, Schmitz, G. P.#, Pedersen, C. E., Marcus, D. J., Shirley, S. E., Hobbs, T. E., Elerding, A. J., Renaud, S. J., Jing, M., Li, Y., Alvarez, V. A., Lemos, J. C., & Bruchas, M. R*. (2021). Ventral tegmental area GABAergic inhibition of cholinergic interneurons in the ventral nucleus accumbens shell promotes reward reinforcement. Nature Neuroscience, 24, 1414–1428
[Full Text] [PDF] |
|
 |
· Farrell, J. S.*, Colangeli, R., Dong, A., George, A. G., Addo-Osafo, K., Kingsley, P. J., Morena, M., Wolff, M. D., Dudok, B., He, K., Patrick, T. A., Sharkey, K. A., Patel, S., Marnett, L. J., Hill, M. N., Li, Y., Teskey, G. C., & Soltesz, I. (2021).
In vivo endocannabinoid dynamics at the timescale of physiological and pathological neural activity. Neuron,, 109(15), 2398-2403.e2394. [Full Text] [PDF] |
|
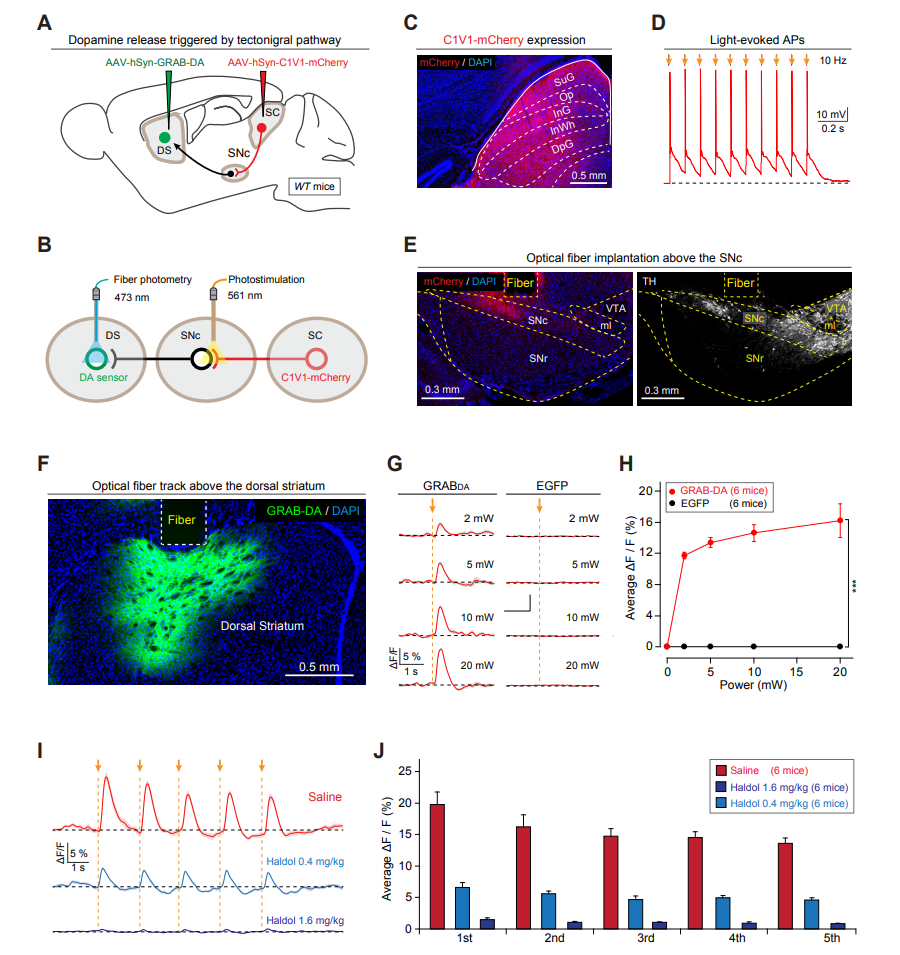 |
· Huang, M.#, Li, D.#*, Pei, Q.#, Xie, Z., Gu, H., Zhang, X., Chen, Z., Liu, A., Wang, Y., Sun, F., Li, Y., Zhang, J., He, M., Xie, Y., Zhang, F., Qi, X., Shang, C.*, & Cao, P.*(2021). The tectonigral pathway regulates appetitive locomotion in predatory hunting in mice Nature Communications, 12, 4409.
[Full Text] [PDF] |
|
 |
· Wang, Q.#, Kong, Y.#, Wu, D., Liu, J., Jie, W., You, Q., Huang, L., Hu, J., Chu, H., Gao, F., Hu, N., Luo, Z., Li, X., Li, S., Wu, Z., Li, Y., Yang, J.*, & Gao, T.* (2021).
Impaired calcium signaling in astrocytes modulates autism spectrum disorder-like behaviors in mice. Nature Communications, 12(1), 3321. [Full Text] [PDF] |
|
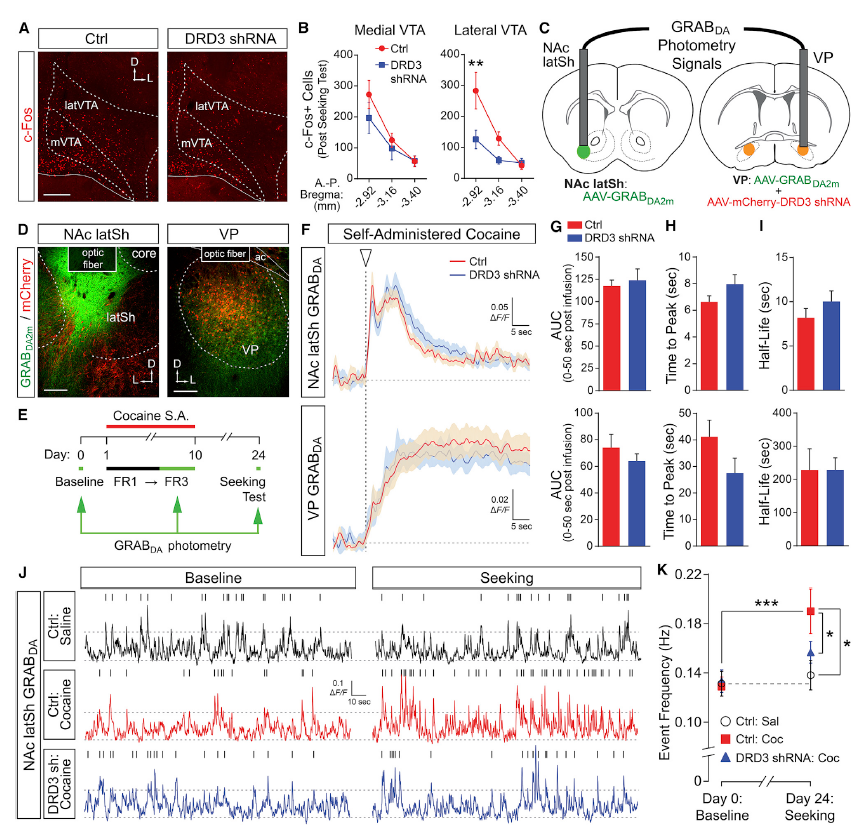 |
· Pribiag, H., Shin, S., Wang, E. H., Sun, F., Datta, P., Okamoto, A., Guss, H., Jain, A., Wang, X. Y., De Freitas, B., Honma, P., Pate, S., Lilascharoen, V., Li, Y., & Lim, B. K.* (2021).
Ventral pallidum DRD3 potentiates a pallido-habenular circuit driving accumbal dopamine release and cocaine seeking. Neuron.
[Full Text] [PDF] |
|
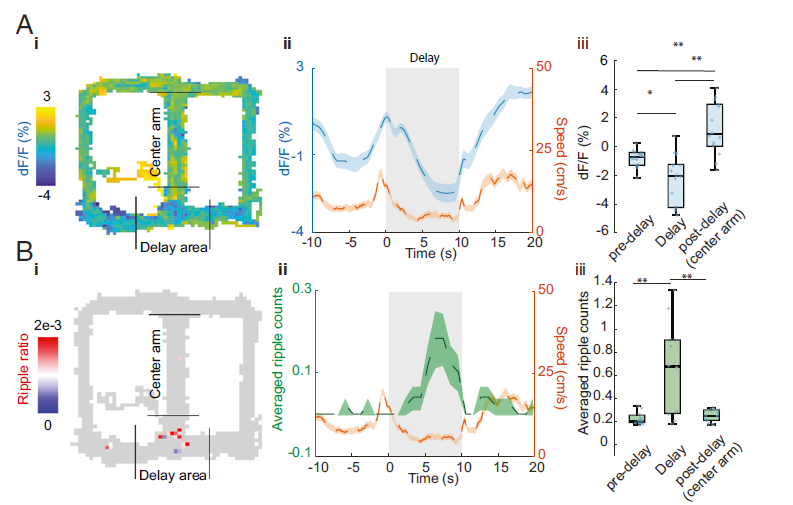 |
· Zhang, Y.#, Cao, L.#, Varga, V., Jing, M., Karadas, M., Li, Y., & Buzsáki, G.* (2021). Cholinergic suppression of hippocampal sharp-wave ripples impairs working memory. Proceedings of the National Academy of Sciences, 118(15), e2016432118. [Full Text] [PDF] |
|
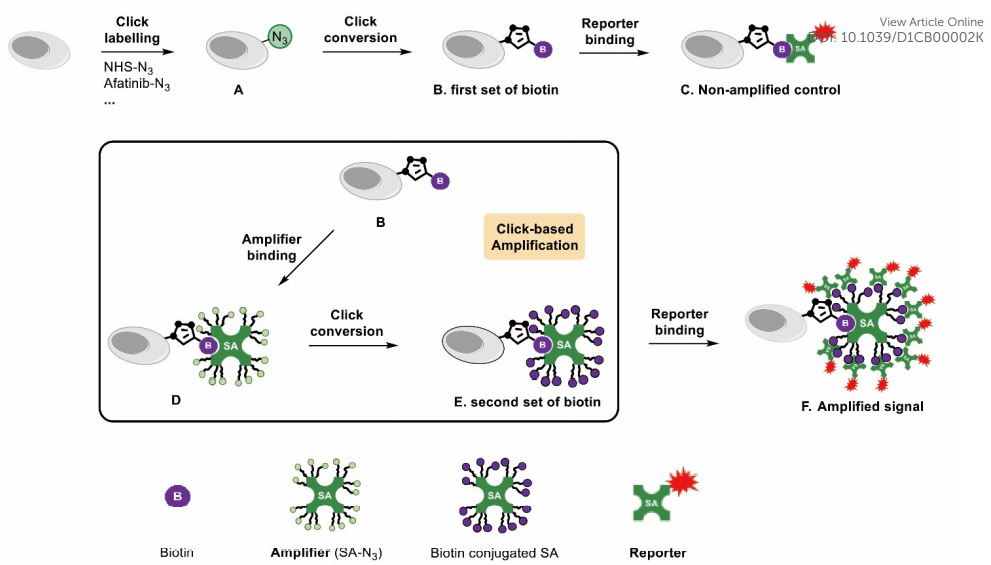 |
· Bai, J., Guo, F., Li, M., Li, Y.*, & Lei, X.* (2021). Click-based amplification: designed to facilitate various target labelling with ultralow background. RSC Chemical Biology,
https://doi.org/10.1039/D1CB00002K. [Full Text] [PDF] |
|
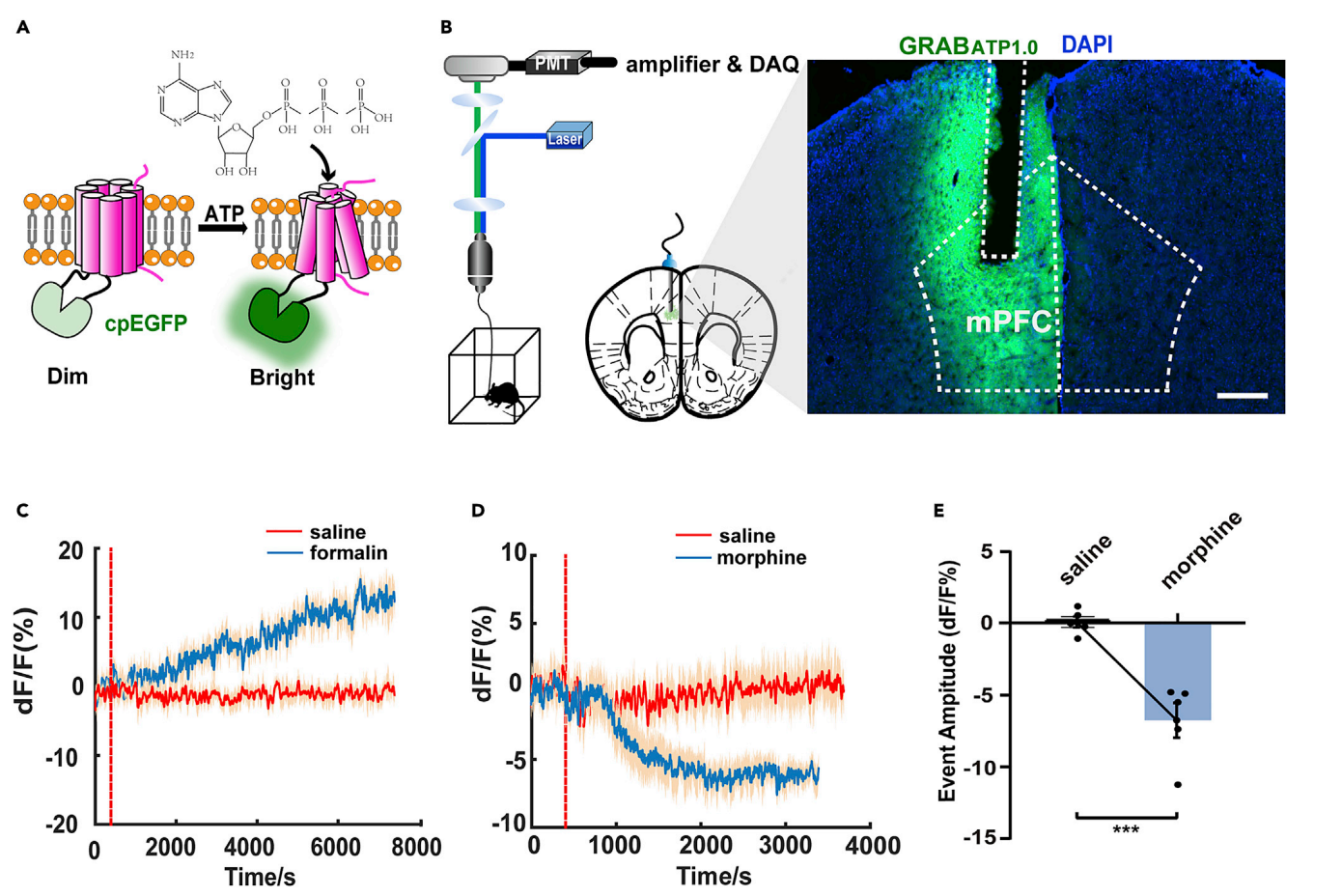 |
· Zeng, Y.#, Luo, H.#, Gao, Z., Zhu, X., Shen, Y., Li, Y., Hu, J.*, & Yang, J.* (2021). Reduction of prefrontal purinergic signaling is necessary for the analgesic effect of morphine. iScience,24(3), 102213. [Full Text] [PDF] |
|
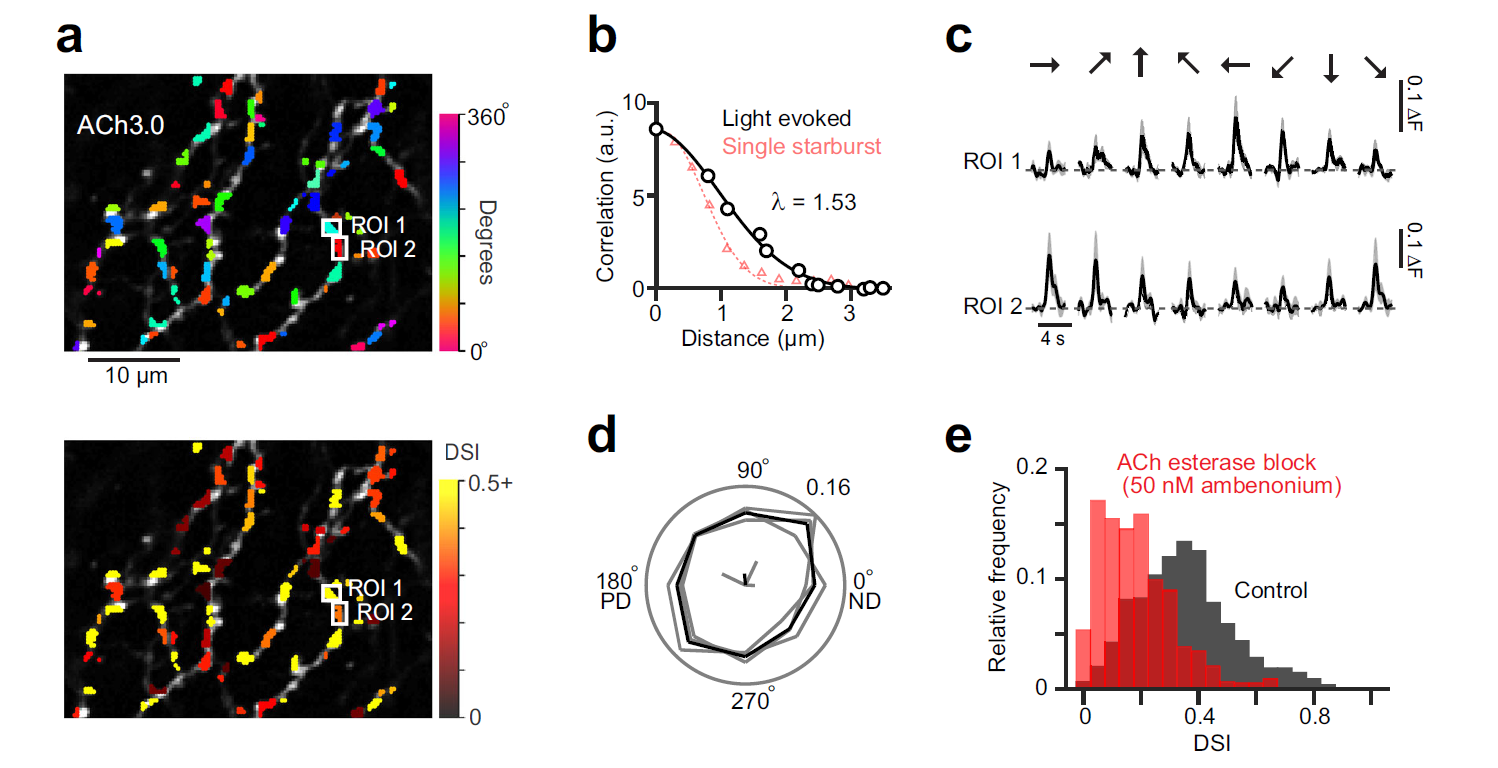 |
· Sethuramanujam, S.#, Matsumoto, A.#, deRosenroll, G., Murphy-Baum, B., McIntosh, J. M., Jing, M., Li, Y., Berson, D., Yonehara, K.*, & Awatramani, G. B.* (2021). Rapid multi-directed cholinergic transmission in the central nervous system. Nature Communications,
[Full Text] [PDF] |
|
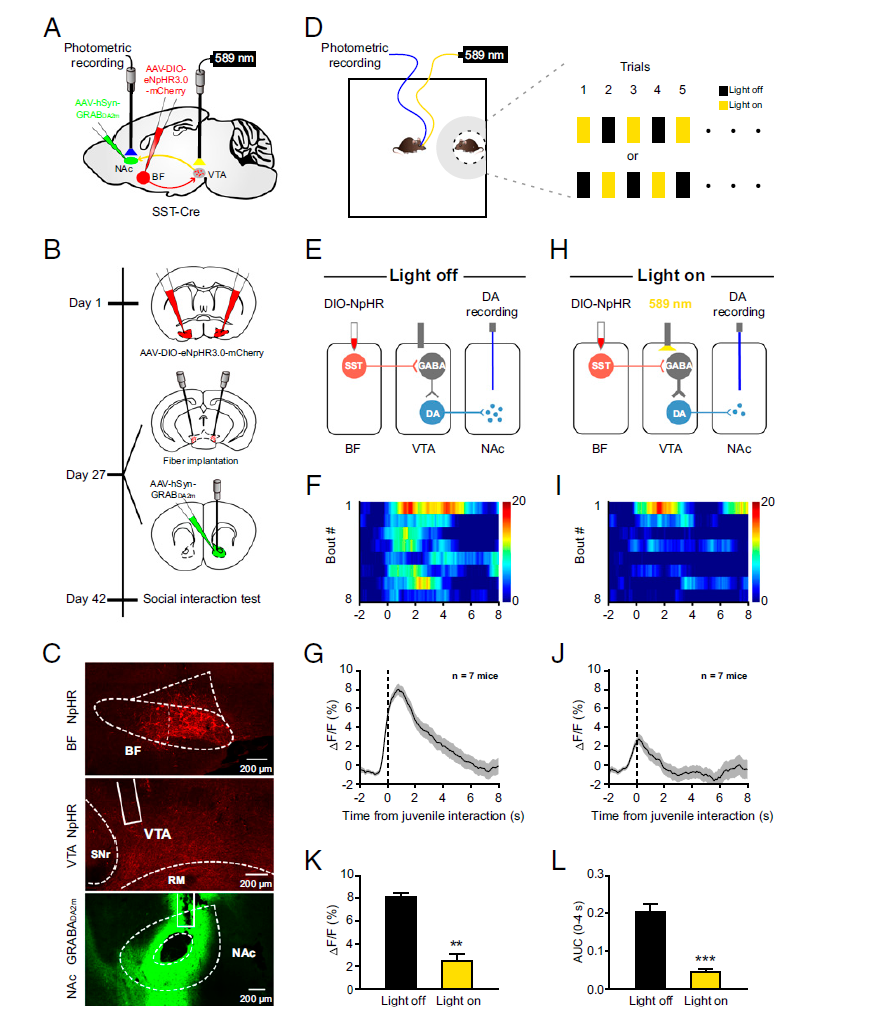 |
· Wang, J.#, Li, J.#, Yang, Q.#, Xie, Y.-K., Wen, Y.-L., Xu, Z.-Z., Li, Y., Xu, T., Wu, Z.-Y., Duan, S., & Xu, H.* (2021). Basal forebrain mediates prosocial behavior via disinhibition of midbrain dopamine neurons. Proceedings of the National Academy of Sciences,118(7), e2019295118. [Full Text] [PDF] |
|
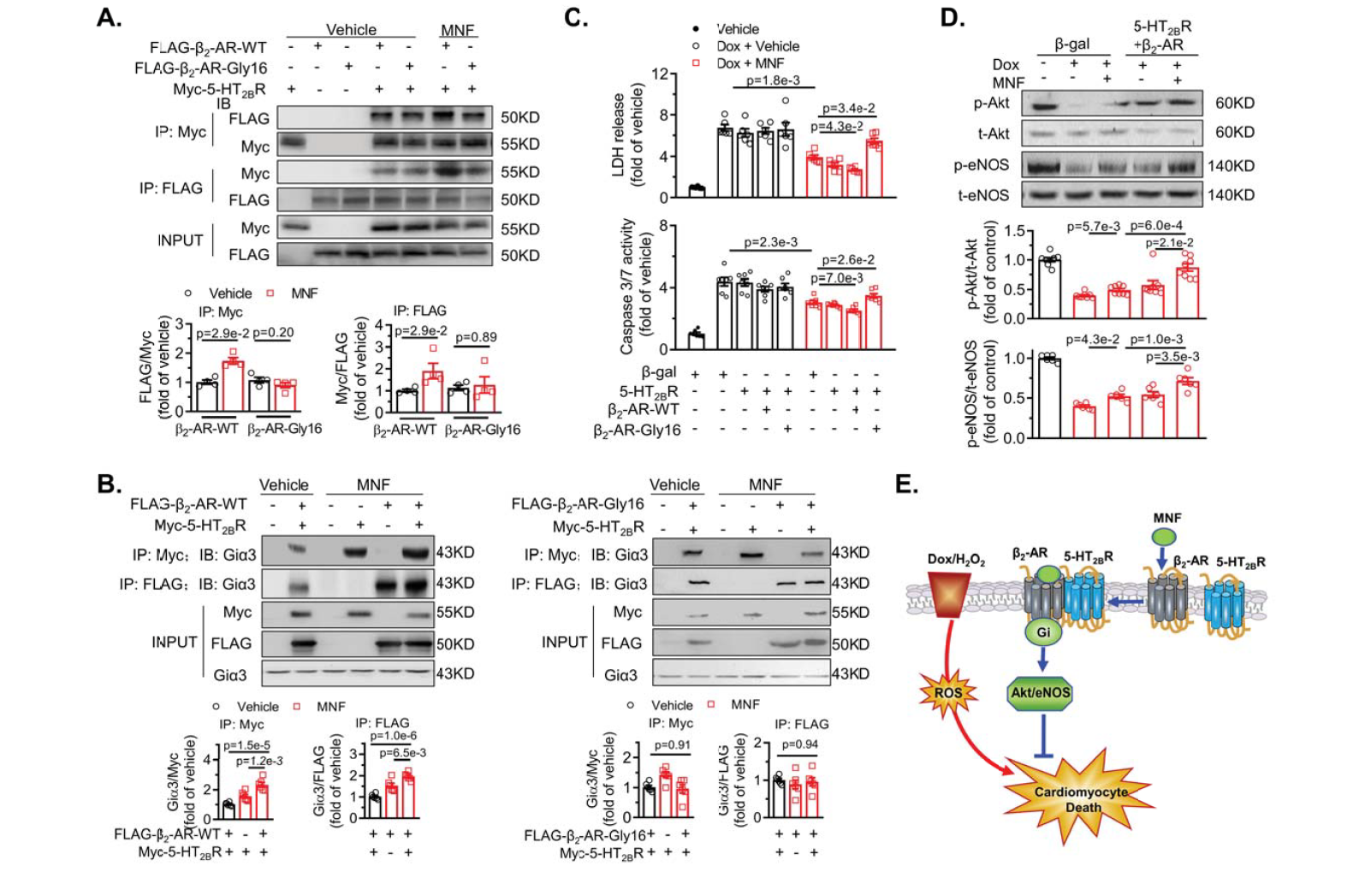 |
· Song, Y., Xu, C., Liu, J., Li, Y., Wang, H., Shan, D., Wainer Irving, W., Hu, X., Zhang, Y.*, Woo Anthony, Y.-H.*, & Xiao, R.-P. Heterodimerization with 5-HT2BR Is Indispensable for β2AR-mediated Cardioprotection. Circulation Research,
[Full Text] [PDF] |
|
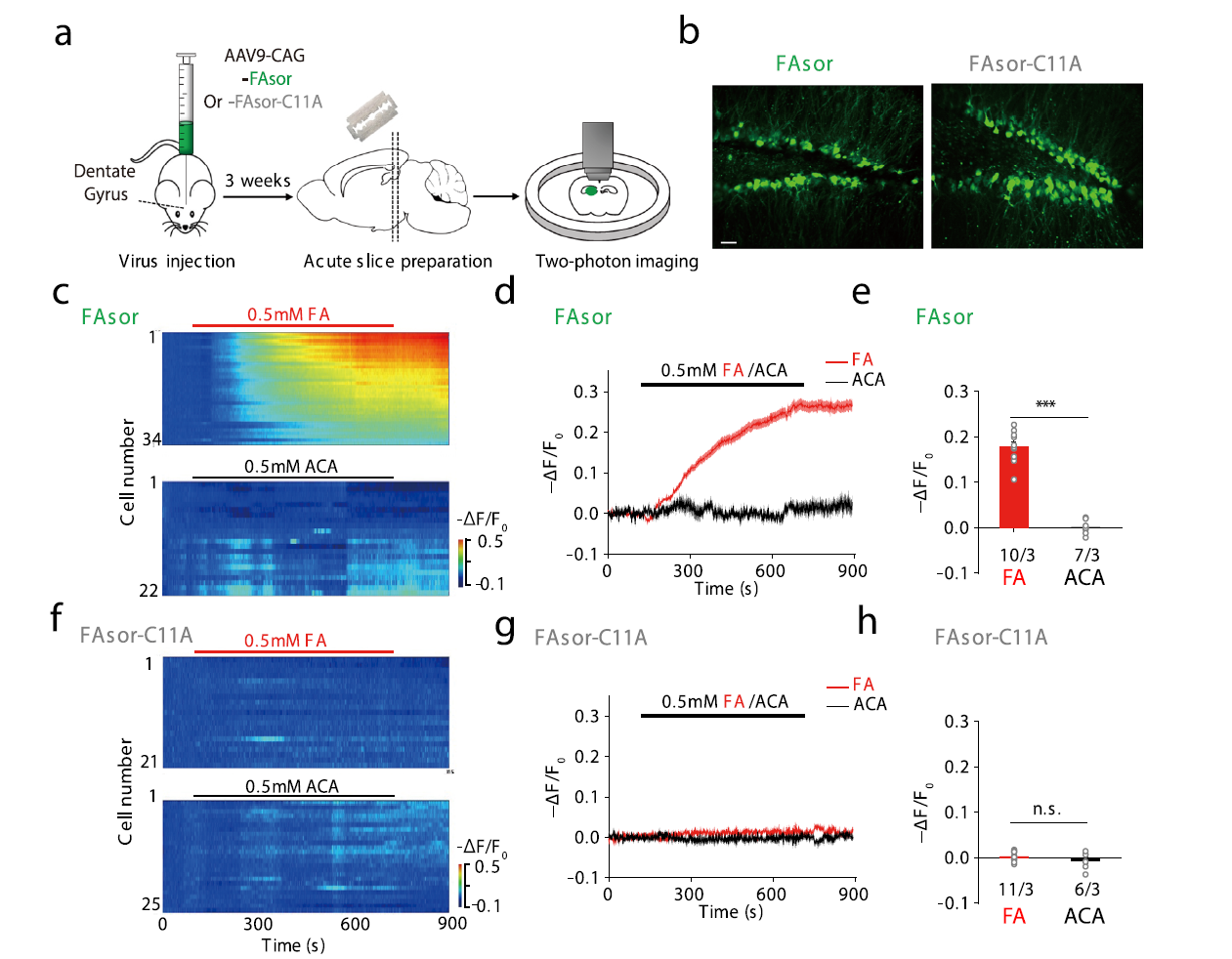 |
· Zhu, R.#, Zhang, G.#, Jing, M., Han, Y., Li, J., Zhao, J., Li, Y., & Chen, P. R.* (2021, 2021/01/25). Genetically encoded formaldehyde sensors inspired by a protein intra-helical crosslinking reaction. Nature Communications,,12(1), 581. [Full Text] [PDF] |
|
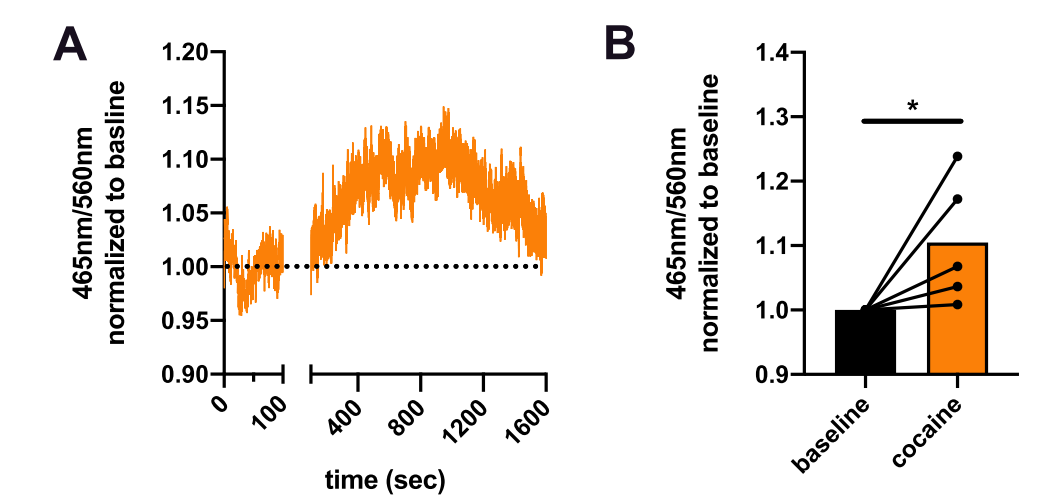 |
· Mayer, F. P., Iwamoto, H., Hahn, M. K., Grumbar, G. J., Stewart, A., Li, Y., & Blakely, R. D.* (2021). There's no place like home? Return to the home cage triggers dopamine release in the mouse nucleus accumbens. Neurochemistry International, 142, 104894. [Full Text] [PDF] |
|
 |
· Bari*, A., Xu, S., Pignatelli, M., Takeuchi, D., Feng, J., Li, Y., & Tonegawa, S.* (2020). Differential attentional control mechanisms by two distinct noradrenergic coeruleo-frontal cortical pathways. Proceedings of the National Academy of Sciences. [Full Text] [PDF] |
|
 |
· Kim, H. R.*, Malik, A. N., Mikhael, J. G., Bech, P., Tsutsui-Kimura, I., Sun, F., Zhang, Y., Li, Y., Watabe-Uchida, M., Gershman, S. J., & Uchida, N.* (2020). A Unified Framework for Dopamine Signals across Timescales. Cell. [Full Text] [PDF] |
|
 |
· Crouse,R. B., Kim, K., Batchelor, H. M., Kamaletdinova, R., Chan, J., Rajebhosale, P., Pittenger, S. T., Role, L. W., Talmage, D A., Jing, M. , Li, Y., Gao, X., Mineur , Y. S., & Picciotto, M. R. * (2020). Acetylcholine is released in the basolateral amygdala in response to predictors of reward and enhances learning of cue-reward contingency. eLife, 9:e57335. [Full Text] [PDF] |
|
 |
· Kwak, H., Koh, W., Kim, S., Song, K., Shin, J., Lee, J. M., Lee, E. H., Bae, J. Y., Ha, G. E., Oh, J. Park, Y. M., Kim, S., Feng, J., Lee, S. E., Choi, J. W., Kim, K. H., Kim, Y. S., Woo, J., Lee, D., Son, T., Kwon, S. W., Park, K. D., Yoon, B. Lee, J., Li, Y. , Lee, H., Bae, Y. C., Lee, C. J.* & Cheong, E.* (2020). Astrocytes Control Sensory Acuity via Tonic Inhibition in the Thalamus. Neuron. [Full Text] [PDF] |
|
 |
· Peng, W.#, Wu, Z.#, Kun, S.#, Zhang, S., Li, Y. & Min, X.* (2020). Regulation of sleep homeostasis mediator adenosine by basal forebrain glutamatergic neurons. Science, 369, 1208. [Full Text] [PDF] |
|
 |
· Mazzone, C.M., Liang-Guallpa, J.,Li, C., Wolcott, N. S., Boone, M. H., Southern, M., Kobzar, N. P., Salgado, I. A., Reddy, D. M., Sun, F., Zhang, Y., Li, Y., Cui, G. * & Krashes, M. J.* (2020). High-fat food biases hypothalamic and mesolimbic expression of consummatory drives. Nature Neuroscience. [Full Text] [PDF] |
|
 |
· DeGroot, S.R., Zhao-Shea, R., Chung L., Klenowski, P.M., Sun, F., Molas, S., Gardner, P.D., Li, Y. & Tapper, A.R.* (2020). Midbrain dopamine controls anxiety-like behavior by engaging unique interpeduncular nucleus microcircuitry. Biological Psychiatry. [Full Text] [PDF] |
|
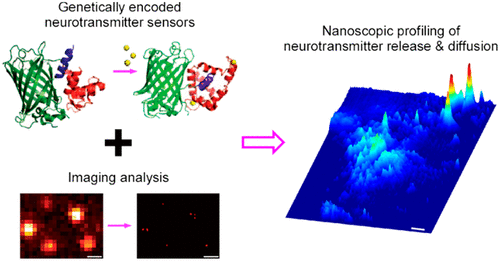 |
· Zhu, P. K. ,Zheng, W. S. , Zhang, P., Jing, M., Borden, P. M., Ali, F., Guo, K., Feng, J., Marvin, J. S., Wang, Y., Wan, J., Gan, L., Kwan, A. C., Lin, L., Looger, L. L., Li, Y. & Zhang, Y.* (2020). Nanoscopic visualization of restricted nonvolume cholinergic and monoaminergic transmission with genetically encoded sensors. Nano Lett.. [Full Text] [PDF] |
|
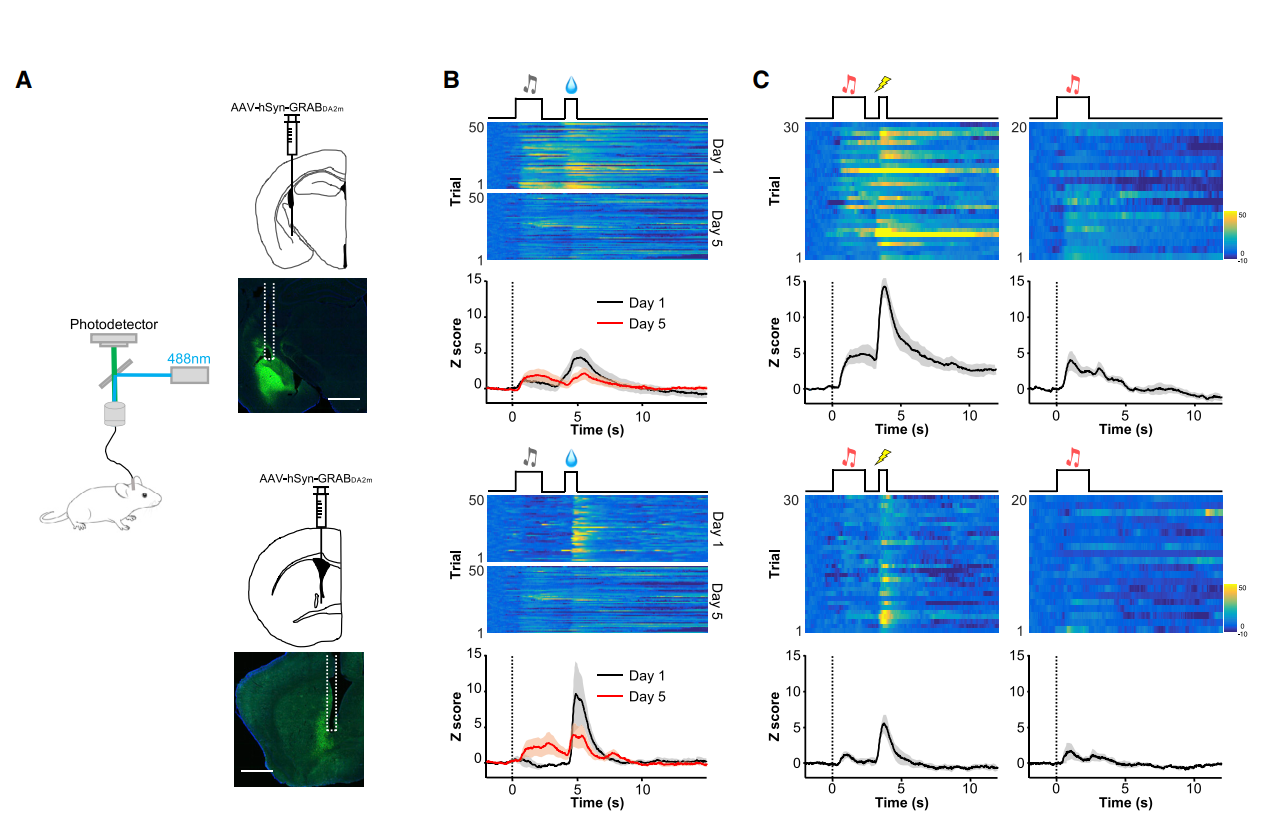 |
· Lin, R.*, Liang, J., Wang, R., Yan, T., Zhou, Y., Liu, Y., Feng, Q., Sun, F., Li, Y., Li, A., Gong, H., & Luo, M.* (2020). The raphe dopamine system controls the expression of incentive memory. Neuron, 1420-19. [Full Text] [PDF] |
|
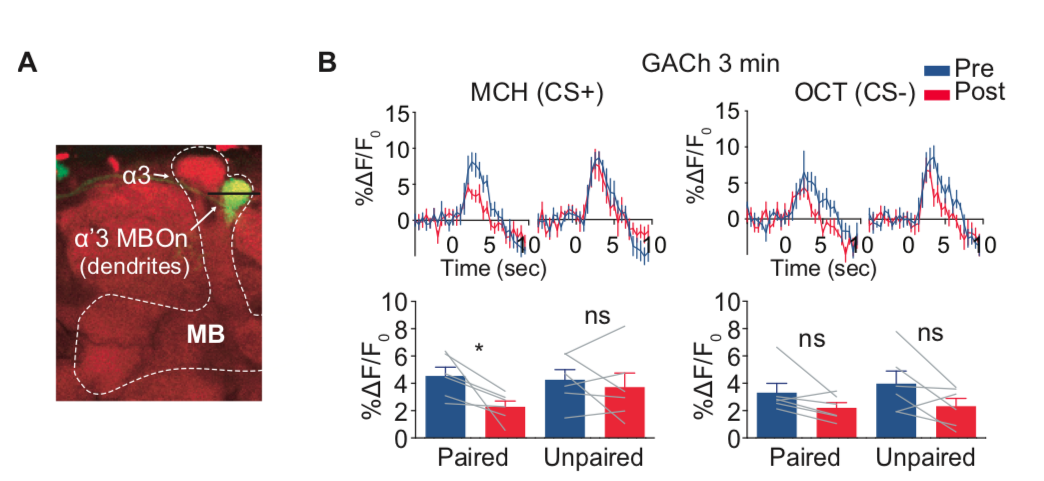 |
· Zhang, X., Noyes, N. C. , Zeng, J., Li, Y. & Davis, R. L.* (2019). Aversive training induces both pre- and postsynaptic suppression in Drosophila. The Journal of Neuroscience, 1420-19. [Full Text] [PDF] |
|
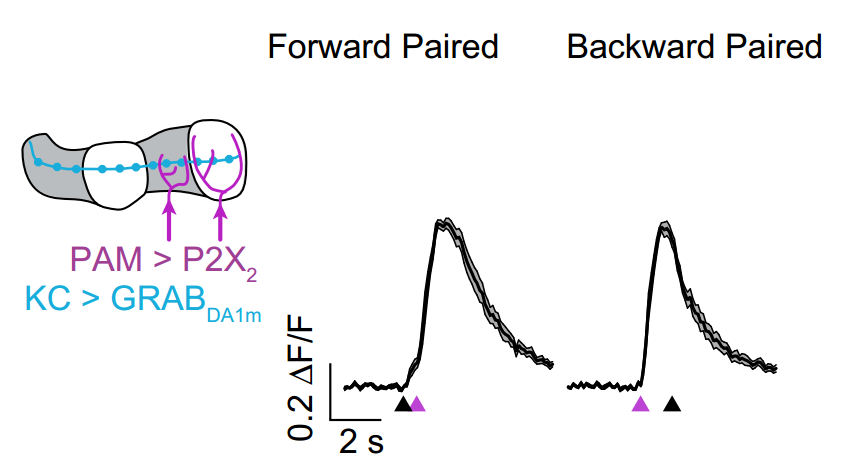 |
· Handler, A., Graham, T. G. M., Cohn, R., Morantte, I., Siliciano, A. F., Zeng J., Li, Y. & Ruta, V.* (2019). Distinct dopamine receptor pathways underlie the temporal sensitivity of associative learning. Cell, 178(1), 60-75. [Full Text] [PDF] |
|
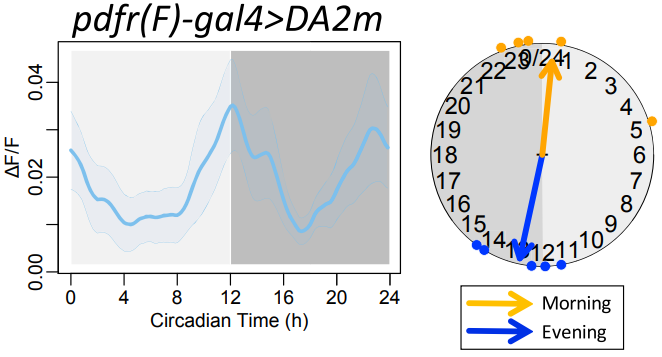 |
· Liang, X., Ho, M. C., Zhang, Y., Li, Y., Wu, M. N., Holy, T. E., & Taghert, P. H.* (2019). Morning and evening circadian pacemakers independently drive premotor centers via a specific dopamine relay. Neuron, 102(4), 843-857. [Full Text] [PDF] |
|
 |
· Zhou, M.#, Chen, N.#, Tian, J., Zeng, J., Zhang, Y., Zhang, X., Guo, J., Sun, J., Li, Y., Guo, A.*, & Li, Y.* (2019). Suppression of GABAergic neurons through D2-like receptor secures efficient conditioning in Drosophila aversive olfactory learning. Proceedings of the National Academy of Sciences, 201812342. [Full Text] [PDF] |
|
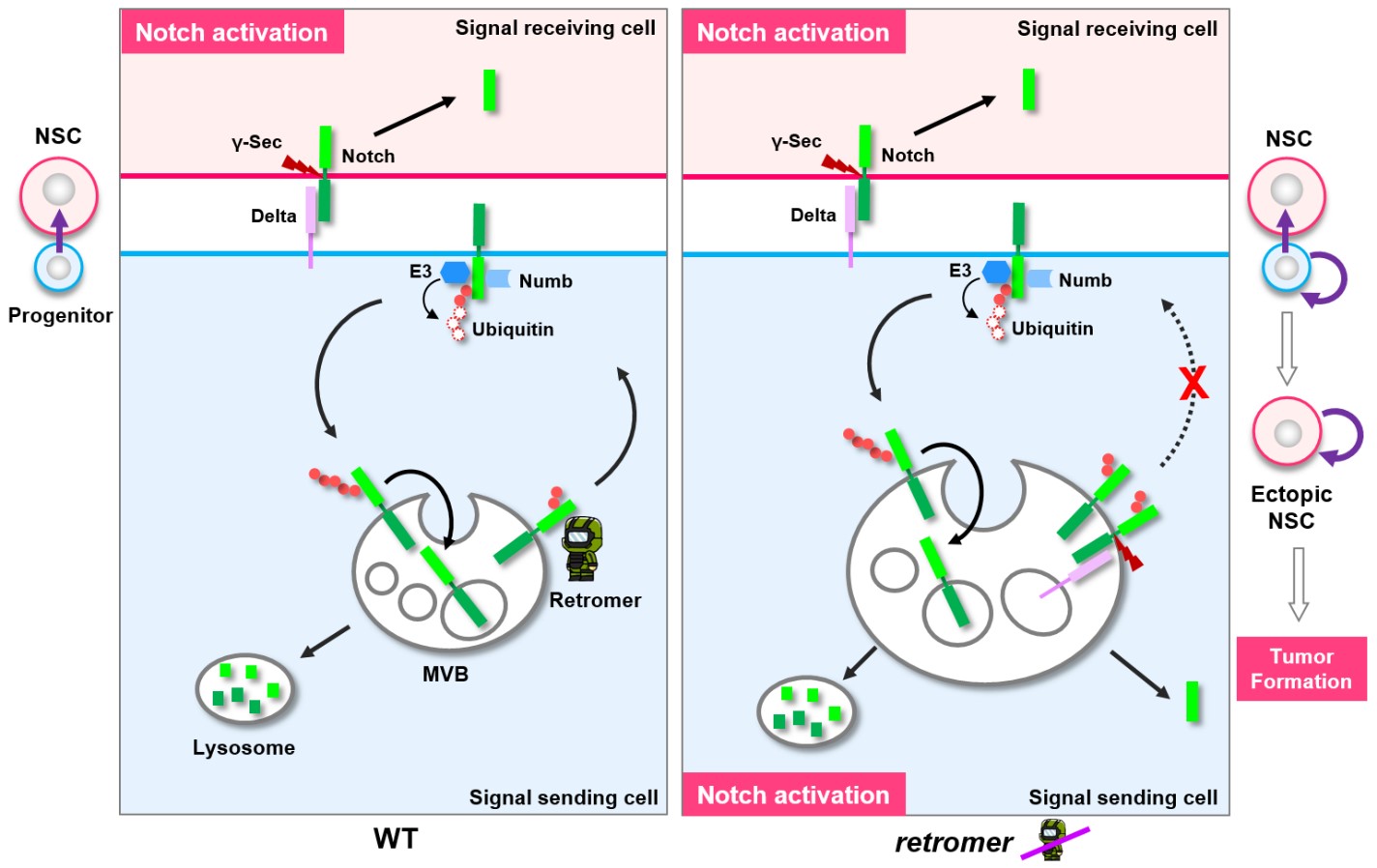 |
· Li, B.#, Wong, C.#, Gao, S. M., Zhang, R., Sun, R., Li, Y., & *Song, Y. (2018). The retromer complex safeguards against neural progenitor-derived tumorigenesis by regulating Notch receptor trafficking. eLife, 7, e38181. [Full Text] [PDF] |
|
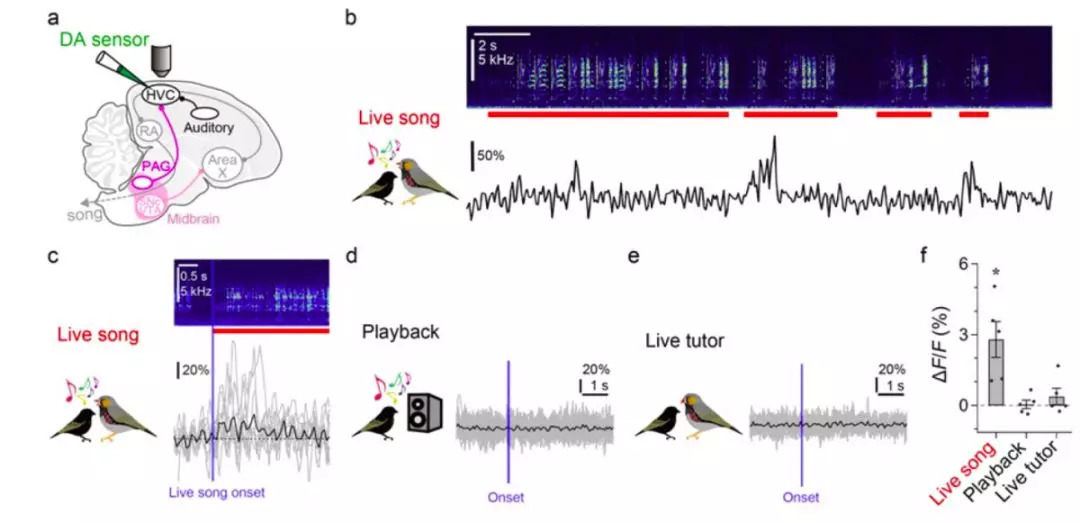 |
· Tanaka, M., Sun, F., Li, Y., & Mooney, R.* (2018). A mesocortical dopamine circuit enables the cultural transmission of vocal behaviour. Nature, 563(7729), 117-120. [Full Text] [PDF][Extended data][Supplementary information] |
|
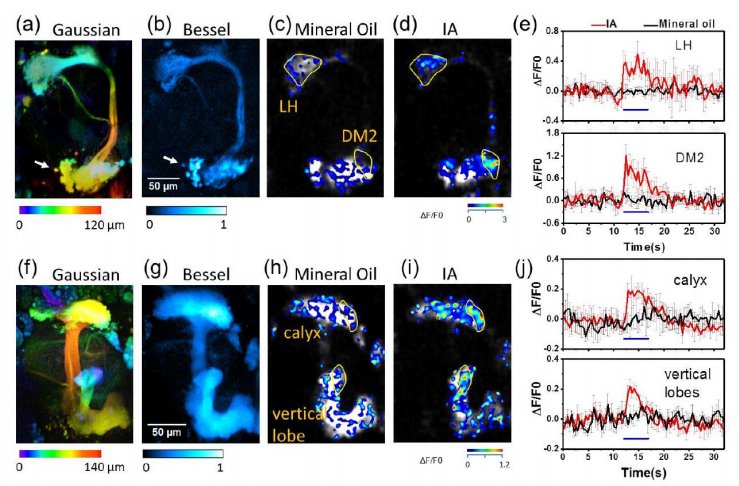 |
· Chen, B.#, Huang, X.#, Gou, D., Zeng, J., Chen , G., Pang, M., Hu, Y., Zhao, Z., Wu, H., Cheng, H., Zhang, Z., Xu, C., & Li, Y., Chen, L.*, Wang, A.* (2018). Rapid volumetric imaging with Bessel-Beam
three-photon microscopy. Biomedical optics express, 9(4), 1992-2000. [Full Text] [PDF] |
|
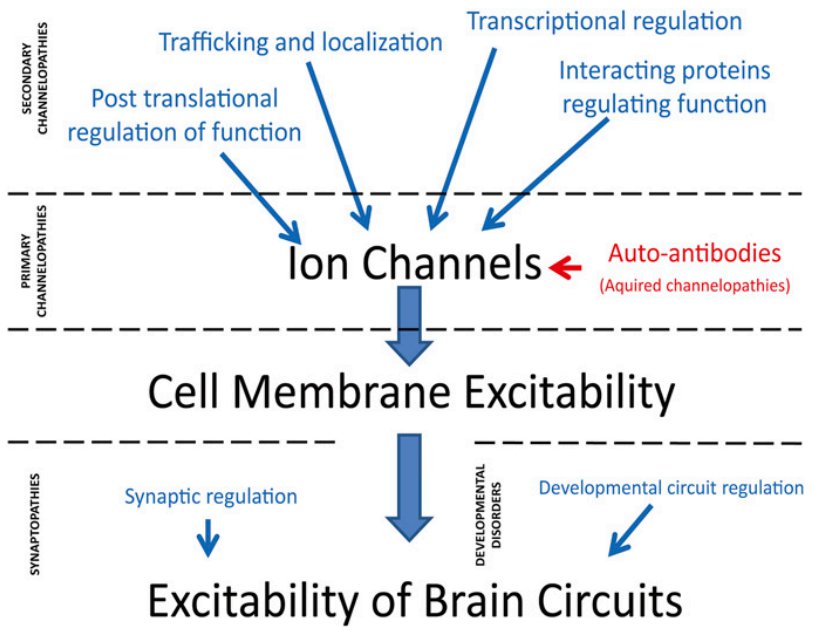 |
· Shen, Y., Ge, W. P., Li, Y., Hirano, A., Lee, H. Y., Rohlmann, A., Missler, M., Tsien, R. W., Jan, L. Y., Fu, Y. H.* & Ptacek, L. J.* (2015). Protein mutated in paroxysmal dyskinesia interacts with the active zone protein RIM and suppresses synaptic vesicle exocytosis. Proceedings of the National Academy of Sciences, 112(10), 2935-2941. [Full Text] [PDF] |
|
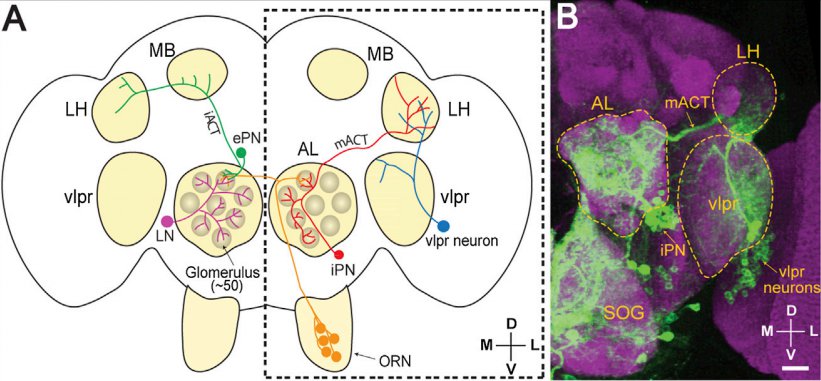 |
· Liang, L., Li, Y., Potter, C. J., Yizhar, O., Deisseroth, K., Tsien, R. W., & Luo, L.* (2013). GABAergic projection neurons route selective olfactory inputs to specific higher-order neurons. Neuron, 79(5), 917-931. [Full Text] [PDF] |
|
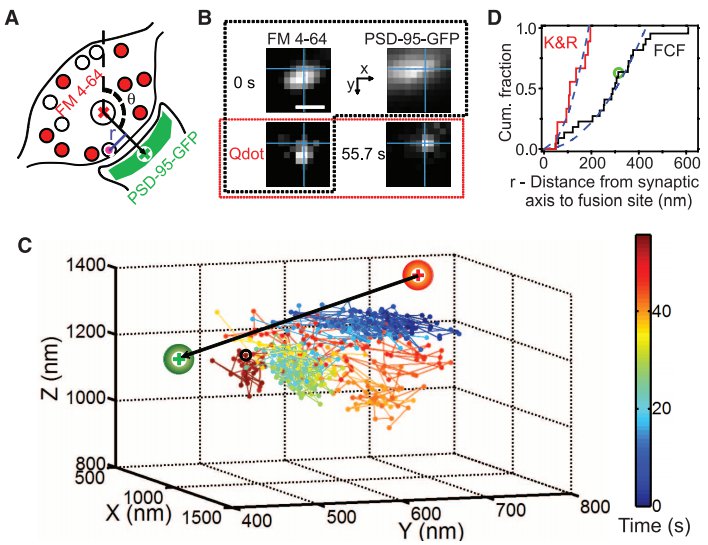 |
· Park, H., Li, Y., & Tsien, R. W.* (2012). Influence of synaptic vesicle position on release probability and exocytotic fusion mode. Science, 335(6074), 1362-1366. [Full Text] [PDF] |
|
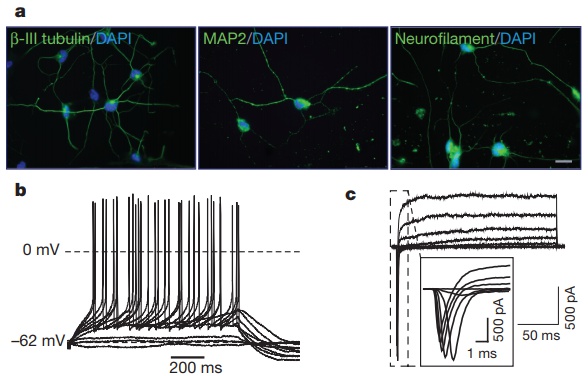 |
· Yoo, A. S.*, Sun, A. X., Li, L., Shcheglovitov, A., Portmann, T., Li, Y., Lee-Messer, C., Dolmetsch, R. E., Tsien R. W. & Crabtree, G. R.* (2011). MicroRNA-mediated conversion of human fibroblasts to neurons. Nature, 476(7359), 228-231. [Full Text] [PDF] |
|
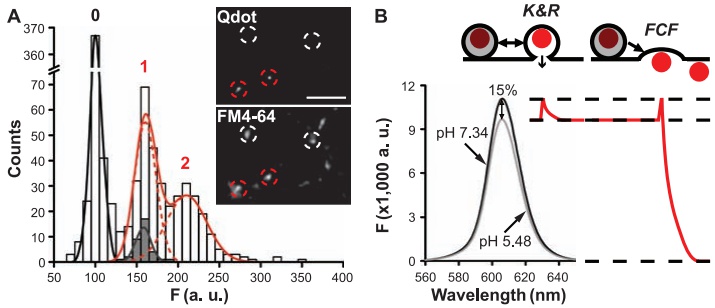 |
· Zhang, Q., Li, Y., & Tsien, R. W.* (2009). The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science, 323(5920), 1448-1453. [Full Text] [PDF] |
|
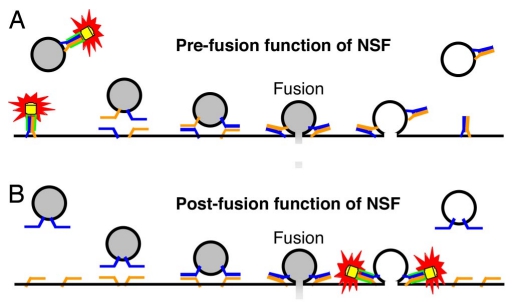 |
· Kuner, T.*, Li, Y., Gee, K. R., Bonewald, L. F., & Augustine, G. J. (2008). Photolysis of a caged peptide reveals rapid action of N-ethylmaleimide sensitive factor before neurotransmitter release. Proceedings of the National Academy of Sciences, 105(1), 347-352. [Full Text] [PDF] |